林鹏 李百涛 刘絮影 邱德强 于小玲


[摘要] 目的 探討姜黄素改善环磷酰胺所致小鼠肠蠕动障碍的作用及机制。方法 选取雄性健康昆明小鼠40只,随机分为对照组、姜黄素组、环磷酰胺组和姜黄素+环磷酰胺组,每组10只。对照组给予溶媒溶液灌胃和生理盐水腹腔注射,姜黄素组给予姜黄素灌胃和生理盐水腹腔注射,环磷酰胺组给予溶媒溶液灌胃和环磷酰胺腹腔注射,姜黄素+环磷酰胺组给予姜黄素灌胃和环磷酰胺腹腔注射。分别于第1次给药前和末次给药后,测量各组小鼠体质量;采用亚甲蓝灌胃法测量小鼠小肠推进率;取小鼠十二指肠组织,采用荧光定量PCR法和Western blot法检测Cajal间质细胞(ICC)相关因子的表达;小鼠十二指肠组织切片后行苏木精-伊红染色观察组织学变化。结果 与对照组相比较,环磷酰胺组小鼠体质量和小肠推进率显著降低(F=112.500、121.988,P<0.01);酪氨酸激酶受体C-kit及其配体干细胞因子(SCF)的mRNA相对表达量(F=181.895、201.84,P<0.01)以及蛋白表达量(F=71.258、93.025,P<0.01)显著下降;组织学观察显示十二指肠黏膜下层大量淋巴细胞浸润。与环磷酰胺组相比较,姜黄素+环磷酰胺组小鼠的体质量和小肠推进率均显著升高(F=19.527、24.511,P<0.01);C-kit和SCF的mRNA相对表达量(F=24.576、37.935,P<0.01)以及蛋白表达量(F=23.631、39.906,P<0.01)显著升高;十二指肠黏膜下层淋巴细胞浸润数量明显减少。而姜黄素组与对照组相比,上述指标差异均无统计学意义(P>0.05)。结论 姜黄素可显著改善环磷酰胺所致小鼠肠蠕动障碍,其机制可能与改善肠组织C-kit/SCF信号有关。
[关键词] 姜黄素;环磷酰胺;胃肠活动;Cajal小肠细胞;小鼠
[中图分类号] R282.71;R333 ?[文献标志码] A ?[文章编号] 2096-5532(2020)02-0185-05
doi:10.11712/jms.2096-5532.2020.56.036 [开放科学(资源服务)标识码(OSID)]
[网络出版] http://kns.cnki.net/kcms/detail/37.1517.r.20200303.1349.007.html;2020-03-04 14:04:59
[ABSTRACT] Objective To investigate the effect of curcumin in improving cyclophosphamide-induced intestinal peristalsis disorder in mice and its mechanism. ?Methods Forty healthy male Kunming mice were selected and randomly divided into four groups (control group, curcumin group, cyclophosphamide group, and curcumin+cyclophosphamide group), with 10 mice in each group. In the control group, solvent solution was intragastrically administered and physiological saline was intraperitoneally injected. In the curcumin group, curcumin was intragastrically administered and physiological saline was intraperitoneally injected. In the cyclophosphamide group, solvent solution was intragastrically administered and cyclophosphamide was intraperitoneally injected. In the curcumin+cyclophosphamide group, curcumin was intragastrically administered and cyclophosphamide was intraperitoneally injected. The body weight of the mice in each group was measured before the initial administration and after the last administration. The small intestinal propulsion rate was determined by gavage with methylene blue. The duodenum tissue was taken from the mice to determine the expression of interstitial cells of Cajal (ICC) related factors using quantitative real-time PCR and Western blot. The duodenum tissue of the mice was made into sections and stained with hematoxylin and eosin to observe the histological changes. ?Results Compared with the control group, the cyclophosphamide group had significantly decreased body weight and small intestinal propulsion rate (F=112.500,121.988;P<0.01), and significantly decreased relative mRNA expression levels of receptor tyrosine kinase (C-kit) and its ligand stem cell factor (SCF) (F=181.895,201.84;P<0.01) and their protein expression levels (F=71.258,93.025;P<0.01); histological observations revealed massive infiltration of lymphocytes in the submucosa of the duodenum. Compared with the cyclophosphamide group, the curcumin+cyclophosphamide group had significantly increased body weight and small intestinal propulsion rate (F=19.527,24.511;P<0.01), and significantly increased relative mRNA expression levels of C-kit and SCF (F=24.576,37.935;P<0.01) and their protein expression levels (F=23.631,39.906;P<0.01); signifi-cantly reduced lymphocyte infiltration in the submucosa of the duodenum was observed. There were no significant differences in theabove indices between the curcumin group and the control group(P>0.05). ?Conclusion Curcumin can significantly improve cyclophosphamide-induced intestinal peristalsis disorder in mice, which may be related to its improving of the C-kit/SCF signaling in the intestinal tissue.
[KEY WORDS] curcumin; cyclophosphamide; gastrointestinal motility; interstitial cells of Cajal; mice
姜黄素是从姜黄的块茎中提取出来的一种天然有效成分[1],作为一种天然的食品添加剂,具有抗炎、抗氧化、抗肿瘤等多种药理作用[2-4]。在胃肠道中,姜黄素在抗肠道炎症和肿瘤方面也起着重要作用[5-9]。环磷酰胺因抗瘤谱较广,对多种类型肿瘤有效,因而是目前临床上常用的抗肿瘤药物[10-11]。但该药的胃肠道副作用较为突出,给病人造成痛苦的同时也影响其临床应用[12]。Cajal间质细胞(ICC)是胃肠运动的起搏器,能够产生自发的慢电波,可以调节胃肠的节律性蠕动[13-15],在正常胃肠运动中起关键作用[16-18]。C-kit是一种酪氨酸激酶受体,是ICC的特异性标志物[19]。C-kit及其配体干细胞因子(SCF)在ICC的分化、发育和功能维持中起着重要的作用[20] 。有研究结果表明,C-kit/SCF信号的不足会导致新生小鼠低氧性肠运动障碍[21]。本实验室前期研究结果显示,姜黄素(200 mg/kg)预先灌胃 10 d以上可显著缓解顺铂引起的小鼠胃肠蠕动障碍[22]。为研究姜黄素是否也能改善其他化疗药物引起的胃肠蠕动障碍,本实验用环磷酰胺腹腔注射制备小鼠胃肠蠕动障碍模型,探讨姜黄素能否改善模型小鼠胃肠蠕动障碍及其机制是否与C-kit/SCF信号通路改变有关。
1 材料和方法
1.1 实验材料
选择雄性健康昆明小鼠(购自中国山东省鲁抗医药股份有限公司实验动物室),体质量为(20±2)g。姜黄素(美国Sigma公司),纯度为99%,使用时将其溶于50 g/L的阿拉伯胶溶液中,配制成质量浓度为25 g/L的混悬液。环磷酰胺(购自中国山东省西亚化学服务有限公司),使用时溶于生理盐水,配制成质量浓度为4 g/L的注射液。RNA提取试剂盒(中国碧云天生物技术有限公司),逆转录PCR(RT-PCR)以及荧光定量PCR(qPCR)试剂盒(购自日本TaKaRa公司);C-kit、SCF、GADPH引物(购自中国生工生物工程有限公司);Western blot凝胶配制试剂盒(中国Solarbio公司);C-kit、SCF、GAPDH一抗(稀释比例均为1∶500)和羊抗兔IgG(H+L)二抗(稀释比例为1∶5 000)均购自中国Bioss生物技术有限公司。1.2 动物分组及处理
小鼠适应性喂养7 d后,随机分为对照组、姜黄素组、环磷酰胺组和姜黄素+环磷酰胺组,每组10只。姜黄素组给予姜黄素(200 mg/kg,0.2 mL)混悬液灌胃12 d,后7 d联合腹腔注射生理盐水,每天1次;环磷酰胺组给予等量阿拉伯胶灌胃12 d,后7 d联合腹腔注射环磷酰胺(100 mg/kg,0.6 mL),每天1次;姜黄素+环磷酰胺组给予等量姜黄素混悬液灌胃12 d,后7 d联合腹腔注射等量环磷酰胺,每天1次;对照组按照上述方式给予阿拉伯胶灌胃和生理盐水腹腔注射。
1.3 小鼠的一般情况观察
观察实验期间小鼠的精神、活动状态、皮毛变化等一般情况;并于注射环磷酰胺的第1天起,记录各组小鼠的体质量,实验结束后,计算每只小鼠用藥前后的体质量变化。
1.4 小肠推进率的测定
各组小鼠末次给药后禁食不禁水24 h,给予800 g/L亚甲蓝混悬液0.8 mL灌胃,20 min后以脊椎脱臼法处死小鼠,测量幽门括约肌至色素最前端的长度以及幽门括约肌至回盲瓣的长度,计算小肠推进率。小肠推进率=(幽门括约肌至色素最前端的长度/幽门括约肌至回盲瓣的长度)×100%。
1.5 qPCR方法检测C-kit和SCF mRNA的相对表达量
取小鼠十二指肠组织40 mg,用离心柱纯化方式提取总RNA,逆转录成cDNA。应用TaKaRa试剂盒以GAPDH作为内参进行qPCR。反应条件为:95 ℃、3 min;95 ℃、10 s,60 ℃、20 s,72 ℃、30 s;共循环40次。每个样本平行检测3管,计算其平均CT值。采用公式2-ΔΔCT分别计算C-kit和SCF mRNA的相对表达量。各种基因扩增的引物序列见表1。
1.6 Western blot检测C-kit和SCF蛋白的表达
取各组小鼠十二指肠组织40 mg置于EP管中,加入蛋白裂解液和蛋白酶抑制剂用高通量组织研磨机研磨,冰上裂解30 min。然后,将其置于低温离心机中,4 ℃下以12 000 r/min离心5 min,采用BCA法检测上清液中蛋白浓度。提取的蛋白在SDS-PAGE凝胶中进行电泳分离,电转至PVDF膜上。将转好的PVDF膜置于50 g/L的牛奶中封闭60 min,加入一抗稀释液(C-kit、SCF、GAPDH均以1∶500稀释),在室温下孵育1 h后4 ℃过夜;次日用1×TBST洗涤3次,再加入二抗稀释液(稀释比例1∶5 000),在室温下孵育1 h,洗膜。用ECL发光染色液显影。使用Image J软件分析条带的灰度值,以GAPDH作为对照,计算出目的蛋白的相对表达量。实验重复3次,取平均值。
1.7 肠组织学观察
取近十二指肠起始部1~2 cm的肠组织,用生理盐水洗净、滤纸吸干,中性甲醛溶液固定,常规石蜡包埋,切片,脱水,苏木精-伊红染色,光镜下观察肠组织的结构改变和淋巴细胞数量。
1.8 统计学处理
应用SPSS 17.0软件进行统计学处理,数据间比较采用析因设计方差分析,以P<0.05为差异有统计学意义。
2 结 ?果
2.1 各组小鼠一般状况及体质量变化
与对照组相比较,环磷酰胺组小鼠精神状态差,活动量明显减少,皮毛粗糙紊乱,体质量明显下降(F=121.988,P<0.01);与环磷酰胺组相比,姜黄素+环磷酰胺组小鼠精神状态有所改善,活动量有所增加,皮毛光滑整齐,体质量下降明显减少(F=19.527,P<0.01);而姜黄素组与对照组相比,小鼠的一般状况和体质量变化差异无统计学意义(P>0.05)。表明姜黄素可以抑制环磷酰胺导致的小鼠体质量下降。见表2。
2.2 各组小鼠小肠推进率的比较
与对照组相比,环磷酰胺组小鼠小肠推进率明显下降(F=112.500,P<0.01);与环磷酰胺组相比,姜黄素+环磷酰胺组小鼠小肠推进率明显升高(F=24.511,P<0.01);而姜黄素组与对照组相比,小鼠小肠推进率并无明显变化(P>0.05)。表明姜黄素可以抑制环磷酰胺导致的小鼠小肠推进率的下降。见表3。
2.3 各组小鼠C-kit和SCF mRNA表达的比较
与对照组相比,环磷酰胺组小鼠肠组织C-kit和SCF mRNA表达减少(F=201.84、181.895,P<0.01);与环磷酰胺组相比,姜黄素+环磷酰胺组小鼠肠组织C-kit和SCF mRNA表达明显增加(F=24.576、37.935,P<0.01);姜黄素组小鼠与对照组相比,C-kit和SCF mRNA的表达差异无统计学意义(P>0.05)。表明姜黄素可以减弱环磷酰胺所致的小鼠十二指肠组织C-kit和SCF mRNA表达降低。见表4。
2.4 各组小鼠C-kit和SCF蛋白表达的比较
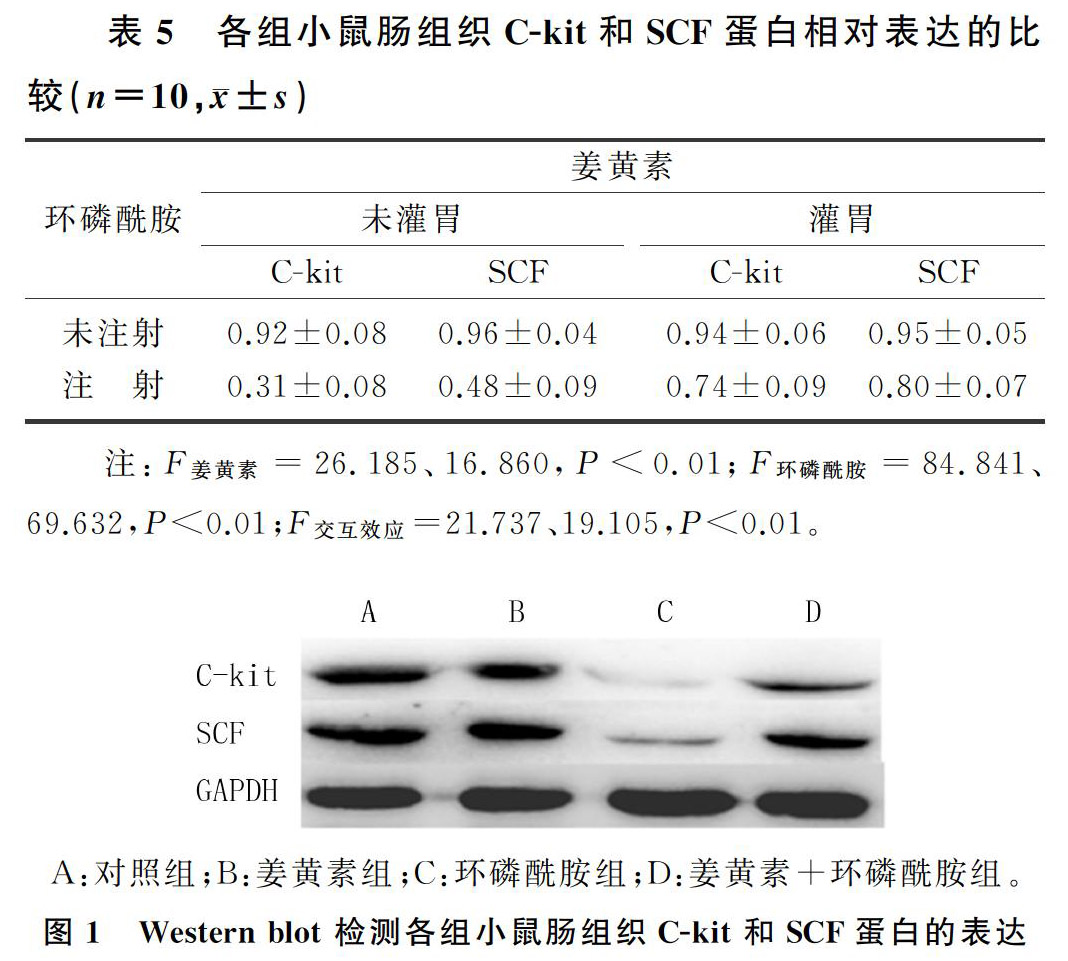
与对照组相比,环磷酰胺组小鼠肠组织C-kit和SCF蛋白的表达明显减少(F=93.025、71.258,P<0.01);姜黄素+环磷酰胺组C-kit和SCF蛋白的表达较环磷酰胺组显著增加(F=39.906、23.631,P<0.01);姜黄素组与对照组相比,两者的表达差异无显著性(P>0.05)。表明姜黄素可以抑制环磷酰胺导致的小鼠十二指肠C-kit和SCF蛋白表达的降低。见表5、图1。
2.5 各组小鼠十二指肠组织学观察
各组小鼠十二指肠均正常,没有结构损伤。与对照组相比,环磷酰胺组小鼠肠黏膜下层可见大量淋巴细胞浸润;姜黄素+环磷酰胺组小鼠肠黏膜下层淋巴细胞数量较环磷酰胺组明显减少;而姜黄素组与对照组相比无明显变化。
3 討 ?论
环磷酰胺是临床上常用的广谱抗肿瘤药物,其产物磷酰胺氮芥对肿瘤细胞和正常细胞的选择性不高,在杀伤肿瘤细胞的同时,对正常组织细胞也有杀伤作用,所以容易产生一定的副作用,尤其是胃肠道副作用[23-24]。本实验通过环磷酰胺腹腔注射制备小鼠肠蠕动障碍模型,可以检测到模型小鼠的肠蠕动较正常小鼠明显减慢,与有关研究结果相似[25]。给予姜黄素预先灌胃可以明显改善环磷酰胺导致的小鼠肠蠕动障碍,这与我实验室前期的研究结果(姜黄素预先灌胃可显著缓解顺铂引起的小鼠胃肠蠕动障碍)相吻合[22],说明姜黄素的确能够缓解不同化疗药物导致的胃肠道蠕动障碍。
以往的研究结果表明,ICC参与动物胃肠道的神经传递[26-30],并且在胃肠功能紊乱中扮演着重要角色[31-34]。C-kit/SCF信号变化能够特异性地反映ICC的功能变化[20]。本文结果显示,环磷酰胺使小鼠十二指肠组织中C-kit及其配体SCF的mRNA和蛋白表达量均明显降低,表明环磷酰胺所致小鼠肠蠕动障碍与肠道ICC的功能下降有关。而姜黄素能减轻环磷酰胺所致的C-kit和SCF表达降低,改善小鼠的胃肠动力。另有研究结果表明,姜黄素可以通过影响C-kit和SCF途径改善糖尿病大鼠的胃轻瘫[35],这和本实验结果相似。至于姜黄素通过何种途径增加C-kit和SCF的表达量,目前尚不清楚,需要进一步的研究。另外,本文苏木精-伊红染色结果显示,环磷酰胺组小鼠十二指肠组织并没有显著的组织结构改变,主要在肠黏膜下层有大量的淋巴细胞浸润。而姜黄素预先干预可使肠黏膜下层淋巴细胞数量明显减少,说明姜黄素也可以抑制肠道的炎症反应。但由于模型小鼠肠组织结构没有明显改变,仅见淋巴细胞浸润,所以姜黄素减轻炎症反应不是其改善小鼠胃肠动力的主要作用机制。
综上所述,在环磷酰胺所致的化疗性胃肠动力障碍模型中,姜黄素可以通过影响C-kit/SCF信号途径来改善小鼠的胃肠蠕动功能,表明姜黄素不仅可辅助抗肿瘤,还可以同时减轻胃肠道副作用。
[参考文献]
[1] BASNIWAL R K, BUTTAR H S, JAIN V K, et al. Curcumin nanoparticles: preparation, characterization, and antimicrobial study[J]. Journal of Agricultural and Food Chemistry, 2011,59(5):2056-2061.
[2] MAHMOOD K, ZIA K M, ZUBER M, et al. Recent developments in curcumin and curcumin based polymeric materials for biomedical applications: a review[J]. International Journal of Biological Macromolecules, 2015,81:877-890.
[3] TSUDA T. Curcumin as a functional food-derived factor: degradation products, metabolites, bioactivity, and future perspectives[J]. Food & Function, 2018,9(2):705-714.
[4] DAILY J W, YANG M, PARK S. Efficacy of turmeric extracts and curcumin for alleviating the symptoms of joint arthritis: a systematic review and meta-analysis of randomized clinical trials[J]. Journal of Medicinal Food, 2016,19(8):717-729.
[5] ZHANG Xingxing, WU Jian, YE Bo, et al. Protective effect of curcumin on TNBS-induced intestinal inflammation is me-diated through the JAK/STAT pathway[J]. BMC Complementary and Alternative Medicine, 2016,16(1):299-309.
[6] SREEDHAR R, ARUMUGAM S, THANDAVARAYAN R A, et al. Curcumin as a therapeutic agent in the chemoprevention of inflammatory bowel disease[J]. Drug Discovery Today, 2016,21(5):843-849.
[7] HUSSAIN Z, THU H E, AMJAD M W, et al. Exploring recent developments to improve antioxidant, anti-inflammatory and antimicrobial efficacy of curcumin: a review of new trends and future perspectives[J]. Materials Science & Engineering C-Materials for Biological Applications, 2017,77:1316-1326.
[8] YUE G G, KWOK H F, LEE J K, et al. Combined therapy using bevacizumab and turmeric ethanolic extract (with absor- ? bable curcumin) exhibited beneficial efficacy in colon cancer mice[J]. Pharmacological Research, 2016,111:43-57.
[9] DULBECCO P, SAVARINO V. Therapeutic potential of curcumin in digestive diseases[J]. World Journal of Gastroente-rology, 2013,19(48):9256-9270.
[10] 陈玲燕,王雪丁,黄民. 环磷酰胺的药物基因组学研究进展[J]. 药学学报, 2014,49(7):971-976.
[11] XIA Qiu, GENG Fei, ZHANG Fangfang, et al. Cyclophosphamide enhances anti-tumor effects of a fibroblast activation protein α-based DNA vaccine in tumor-bearing mice with murine breast carcinoma[J]. Immunopharmacology and Immunotoxicology, 2017,39(1):37-44.
[12] 王穎,刘晓平,周娟. 甲氧氯普胺给药时机对环磷酰胺所致胃肠道症状影响的观察[J]. 解放军护理杂志, 2004,21(11):22.
[13] WEI R H, PARSONS S P, HUIZINGA J D. Network properties of interstitial cells of Cajal affect intestinal pacemaker activity and motor patterns, according to a mathematical model of weakly coupled oscillators[J]. Experimental Physiology, 2017,102(3):329-346.
[14] SATHAR S, TREW M L, CHENG L K. Tissue specific si-mulations of interstitial cells of cajal networks using unstructured meshes[J]. International Conference of the IEEE Engineering in Medicine and Biology Society, 2015,2015:8062-8065.
[15] 陈健海,仲婕,王凡,等. 胃肠道Cajal间质细胞起搏功能的研究进展[J]. 中国病理生理杂志, 2017,33(1):184-188.
[16] KITO Y, MITSUI R, WARD S M, et al. Characterization of slow waves generated by myenteric interstitial cells of Cajal of the rabbit small intestine[J]. American Journal of Physiology-Gastrointestinal and Liver Physiology, 2015,308(5):G378-G388.
[17] HAGGER R, FINLAYSON C, JEFFREY I, et al. Role of the interstitial cells of Cajal in the control of gut motility[J]. The British Journal of Surgery, 1997,84(4):445-450.
[18] PASTERNAK A, SZURA M, GIL K, et al. Interstitial cells of Cajal (ICC)-systematic review[J]. Folia Morphologica, 2016,75(3):281-286.
[19] TAMADA H, KIYAMA H. Existence of c-Kit negative cells with ultrastructural features of interstitial cells of Cajal in the subserosal layer of the W/Wv mutant mouse colon[J]. Journal of Smooth Muscle Research, 2015,51:1-9.
[20] TAN Yuyan, JI Zhenling, HAO Gang, et al. Decreased SCF/c-kit signaling pathway contributes to loss of interstitial cells of Cajal in gallstone disease[J]. International Journal of Clinical and Experimental Medicine, 2014,7(11):4099-4106.
[21] REN Hong, HAN Juan, LI Zhifang, et al. Stem cell factor/kit signal insufficiency contributes to hypoxia-induced intestinal motility dysfunctions in neonatal mice[J]. Digestive Diseases and Sciences, 2017,62(5):1193-1203.
[22] 李培杰,王红伟,刘艳婷,等. 不同天数姜黄素灌胃对顺铂所致小鼠胃排空障碍的作用[J]. 现代生物医学进展, 2016,15(12):2852-2854.
[23] 赵培培,禚映辰,赵雪,等. 环磷酰胺在节律性化疗中的研究进展[J]. 中国医院药学杂志, 2018,38(1):104-108.
[24] 曹露. 鱿鱼墨多糖对环磷酰胺所致小鼠肠道黏膜上皮细胞损伤的保护作用研究[D]. 青岛:中国海洋大学, 2013:74-77.
[25] 弓淑珍,陈宝田,谢炜,等. 枳实消痞汤防治化疗性胃肠功能障碍作用研究[J]. 中药药理与临床, 2006,22(1):14-15.
[26] WANG L L, LIANG Y, CHEN Q S, et al. Identification and distribution of the interstitial cells of cajal in the abomasum of goats[J]. Cell Transplantation, 2018,27(2):335-344.
[27] 陈鹏德,王宝西. Cajal间质细胞相关研究进展[J]. 中华实用儿科临床杂志, 2016,31(19):1506-1508.
[28] BLAIR P J, RHEE P L, SANDERS K M, et al. The significance of interstitial cells in neurogastroenterology[J]. Neurogastroenterol Motil, 2014,20(3):294-317.
[29] SANDERS K M, SALTER A K, HENNIG G W, et al. Responses to enteric motor neurons in the gastric fundus of mice with reduced intramuscular interstitial cells of Cajal[J]. Journal of Neurogastroenterology & Motility, 2014,20(2):171-184.
[30] GOYAL R K, CHAUDHURY A. Mounting evidence against the role of ICC in neurotransmission to smooth muscle in the gut[J]. American Journal of Physiology, Gastrointestinal and Liver Physiology, 2010,298(1):10-13.
[31] 程阔菊,罗云,彭雷,等. Cajal间质细胞在胃肠功能紊乱中的研究进展[J]. 胃肠病学和肝病学杂志, 2015,24(6):749-750.
[32] 黄振鹏,邱虎,徐保平,等. Cajal间质细胞在消化系统动力性疾病发病中作用机制的研究进展[J]. 国际消化病杂志, 2018,38(1):28-31.
[33] 童卫东,刘宝华. Cajal间质细胞与结肠动力紊乱[J]. 中国现代普通外科进展, 2016,10(10):189-191.
[34] 贺巍,朱琳,范兴爱,等. 快速进入高海拔地区小肠动力紊乱大鼠Cajal间质细胞及P物质表达的变化[J]. 胃肠病学和肝病学杂志, 2017,26(3):275-278.
[35] JIN Qihui, SHEN Hongxia, WANG Hui, et al. Curcumin improves expression of SCF/c-kit through attenuating oxidative stress and NF-κB activation in gastric tissues of diabetic gast-roparesis rats[J]. Diabetology & Metabolic Syndrome, 2013,5(1):12-24.
(本文編辑 马伟平)
- 民间舞蹈课堂教学的改革策略分析
- “审美与快乐”式的小学音乐教学策略
- 钢琴教学中对演奏技巧的运用探讨
- 基于职业生涯规划为核心创新就业课程建设研究
- 互联网+时代高职大学生创新创业思路研究
- 教学体会之歌唱的共鸣
- 将定格动画融入儿童美术课程的创新性研究
- “项目引导”类课程的改革与实践
- 微课理念在大学英语教学中的应用
- 关于钢琴课程与音乐教学活动的对接策略
- 高师院校教师口语课程教学改革研究
- 中华传统文化(京剧)进高校研究
- 论民俗文化在汉语国际教育中的传播与应用
- 医学类高职高专如何开展校外传统文化教育
- 越剧《祥林嫂》与鲁迅《祝福》对比分析
- 宁波甬剧的动漫化传承与传播途径研究
- 从羌戏与朵翀看戏剧从宗教仪式脱胎
- 企业在新经济形势下如何创新管理模式
- 杜拉斯《情人》中“中国情人”形象的文化解读
- 文体学视角下《姐妹们》的对话分析
- 论博物馆藏品保管部门的职能
- 接受美学视角下的甬菜菜名翻译研究
- 《堂吉诃德》对现代小说的影响
- 五溪流域古村镇艺术生态探研
- 民族民间艺术在文化创意中传承
- sued
- suede
- suedeing
- suedene
- suedes
- suedine
- sueding
- suer
- suers
- sues
- suet
- suetier
- suetiest
- suets
- suffer
- marketingcommunications
- marketing concept
- marketingconcept
- marketingenvironment
- marketing enˌvironment
- marketing mix
- marketingmix
- marketing myopia
- marketingmyopia
- marketingorientation
- 革命摇篮
- 革命文学
- 革命无产阶级最好的机关报
- 革命样板戏
- 革命洪流
- 革命派
- 革命烈士
- 革命熔炉
- 革命现代戏
- 革命的代数学
- 革命的力量
- 革命的标志
- 革命的气势
- 革命的金娃
- 革命空军之父
- 革命者决不怕批判自己
- 革命者,应乎天,顺乎人
- 革命英雄主义
- 革命风暴
- 革命风雷
- 革囊
- 革囊朝阳
- 革囊鞶囊
- 革图易虑
- 革大