王珊 冯文静 毛拥军


[摘要] 目的 探讨褐藻胶寡糖(AOS)对D-半乳糖(D-gal)诱导的衰老小鼠骨质疏松的作用及其可能的机制。
方法 将45只8周龄雄性C57BL/6J小鼠随机分为对照组(Control组)、模型组(D-gal组)、D-gal+AOS低剂量组(D-gal+AOS-L组)、D-gal+AOS中剂量组(D-gal+AOS-M组)和D-gal+AOS高剂量组(D-gal+AOS-H组)。除Control组外,其余组小鼠颈背部注射D-gal 8周建立小鼠骨质疏松模型。D-gal+AOS-L、D-gal+AOS-M和D-gal+AOS-H组从第5周开始分别给予50、100、150 mg/(kg·d)的AOS灌胃4周,Control组和D-gal组给予蒸馏水灌胃。药物干预完成后,应用骨密度仪测量各组小鼠股骨骨密度,采用Western Blot检测小鼠股骨组织中衰老相关蛋白P16及氧化应激相关蛋白P67 phox的表达,RT-PCR检测氧化应激相关基因p47 phox和gp91 phox mRNA的表达及破骨细胞活化相关基因核因子κB受体活化因子配体(RANKL)mRNA的表达。
结果 D-gal组小鼠骨密度较Control组降低,AOS干预各组小鼠骨密度较D-gal组均增加,差异有统计学意义(F=46.853,P<0.05)。D-gal組小鼠股骨组织中P16和P67 phox蛋白表达较Control组增加,AOS干预各组P16和P67 phox蛋白表达较D-gal组降低,差异均有统计学意义(F=50.862、156.943,P<0.05)。D-gal组小鼠股骨组织中p47 phox、gp91 phox和RANKL mRNA相对表达量较Control组增加,AOS干预各组小鼠p47 phox、gp91 phox和RANKL mRNA相对表达量较D-gal组均降低,差异有统计学意义(F=17.373~112.311,P<0.05)。
结论 AOS对 D-gal诱导的小鼠骨质疏松具有保护作用,其机制可能与氧化应激和破骨细胞活化的抑制有关。
[关键词] 海藻酸;寡糖类;半乳糖;骨质疏松;衰老;氧化性应激;RANK配体
[中图分类号] R681
[文献标志码] A
[文章编号] 2096-5532(2020)03-0261-04
doi:10.11712/jms.2096-5532.2020.56.113
[开放科学(资源服务)标识码(OSID)]
[网络出版] https://kns.cnki.net/kcms/detail/37.1517.R.20200610.1359.004.html;2020-06-11 11:06
EFFECT OF ALGINATE OLIGOSACCHARIDE ON D-GALACTOSE-INDUCED OSTEOPOROSIS IN MICE
WANG Shan, FENG Wenjing, MAO Yongjun
(Department of Geriatric Medicine, The Affiliated Hospital of Qingdao University, Qingdao 266100, China)
[ABSTRACT]\ Objective\ To investigate the effect of alginate oligosaccharide (AOS) on D-galactose (D-gal)-induced osteoporosis in senescent mice and its possible mechanism.
Methods\ A total of 45 male C57BL/6J mice, aged 8 weeks, were randomly divided into Control group, model group (D-gal group), D-gal+low-dose AOS group (D-gal+AOS-L group), D-gal+middle-dose AOS group (D-gal+AOS-M group), and D-gal+high-dose AOS group (D-gal+AOS-H group). All mice except those Control group were given subcutaneous injection of D-gal at the back of the neck for 8 weeks to establish a mouse model of osteoporosis. D-gal+AOS-L group, D-gal+AOS-M group, and D-gal+AOS-H group were given AOS by gavage at a dose of 50, 100, and 150 mg/(kg·d) for 4 weeks since week 5, and Control group and D-gal group were given distilled water by gavage. After drug intervention, dual-energy X-ray absorptiometry was used to measure bone mineral density (BMD) of the femur, Western Blot was used to measure the protein expression of the aging-related protein P16 and the oxidative stress-related protein P67 phox, and RT-PCR was used to measure the mRNA expression of the oxidative stress-related genes p47 phox and gp91 phox and the osteoclast activation-related gene receptor activator of nuclear factor-kappa B ligand (RANKL).
\ Results\ Compared with Control group, D-gal group had a significant reduction in BMD, and compared with D-gal group, the AOS intervention groups had a significant increase in BMD (F=46.853,P<0.05). Compared with Control group, D-gal group had significant increases in the protein expression of P16 and P67 phox in femoral tissue, and compared with D-gal group, the AOS intervention groups had significant reductions in the protein expression of P16 and P67 phox (F=50.862,156.943;P<0.05). Compared with Control group, D-gal group had significant increases in the relative mRNA expression of p47 phox, gp91 phox, and RANKL in femoral tissue, and compared with D-gal group, the AOS intervention groups had significant reductions in the relative mRNA expression of p47 phox, gp91 phox, and RANKL (F=17.373-112.311,P<0.05).
\ Conclusion
AOS exerts a protective effect against D-gal-induced osteoporosis in
mice, possibly by inhibiting oxidative stress and osteoclast activation.
骨质疏松具有骨微观结构退行性改变、骨量减少和骨密度降低的特点,导致骨强度降低[1]、骨脆性增加[2]及骨折风险增加[3],引起了老龄化社会的广泛关注。氧化应激学说是衰老的重要机制之一[4],该学说认为活性氧(ROS)的积聚超过机体的清除能力造成DNA损伤、激活氧化应激,从而导致衰老及衰老相关疾病的发生。ROS在体内积聚可激活核因子κB受体活化因子配体(RANKL)信号通路,导致骨吸收增加。RANKL信号通路被认为是促进破骨细胞活化的主要靶点,可激活多种下游信号通路[5],从而促进骨吸收和破骨细胞分化过程。长期注射D-半乳糖(D-gal)可导致实验动物产生一系列类似于自然老化的病理变化,如认知障碍、氧化应激和骨量减少等[6]。用D-gal诱导建立亚急性衰老模型具有周期短、价格低廉、操作简便、结果稳定可靠等优点,已广泛应用于衰老机制研究和抗衰老药物筛选。褐藻胶寡糖(AOS)具有较高的生物活性,如抗炎、抗氧化、抗凋亡、抗肿瘤等[7]。骨质疏松性骨折是导致老年人死亡的主要原因之一[8],因此骨質疏松症的早期预防、诊断和治疗十分重要,开发更有效的延缓骨质疏松症的药物具有重要意义。目前大多数骨质疏松研究侧重于绝经后妇女,而缺乏对老年男性骨质疏松的研究。故本实验采用D-gal诱导建立小鼠骨质疏松模型,探讨AOS对衰老雄性小鼠骨质疏松的作用及其可能的机制。
1 材料和方法
1.1 实验材料
健康雄性C57BL/6J小鼠45只,SPF级,8周龄,购自济南朋悦实验动物繁育有限公司,饲养于青岛大学医学部SPF级实验动物中心。饲养条件:12 h昼夜循环,室温(20±2)℃,相对湿度40%~60%,自由摄食物和水。动物实验操作均经青岛大学动物福利和伦理管理委员会批准,并且遵守中国动物保护委员会制订的《动物保护与使用指南》。P16小鼠单克隆抗体购自美国 Cell Signaling Technology公司,RIPA裂解液和BCA蛋白浓度测定试剂盒购自上海碧云天公司,逆转录试剂盒及Mix购自Roche公司。
1.2 实验方法
1.2.1 动物分组及处理 45只小鼠适应性喂养1周后,随机分为对照组(Control组,A组)、模型组(D-gal组,B组)、D-gal+AOS低剂量组(D-gal+AOS-L组,C组)、D-gal+AOS中剂量组(D-gal+AOS-M组,D组)以及D-gal+AOS高剂量组(D-gal+AOS-H组,E组),每组9只。Control组小鼠颈背部皮下注射灭菌注射用水5 mL/(kg·d),其余组小鼠颈背部皮下注射D-gal 200 mg/(kg·d),连续8周。从D-gal注射第5周开始,AOS干预低、中、高剂量组分别给予AOS 50、100、150 mg/(kg·d)灌胃处理4周,Control组和D-gal组小鼠给予蒸馏水10 mL/(kg·d)灌胃处理4周。
1.2.2 骨密度测量 药物干预完成后,取每组各3只小鼠的同侧股骨,采用双能X线骨密度仪(Dexa;Osteosys Primus,Korea)对整个股骨进行骨密度测量(扫描间距1.5 mm,扫描速度60 mm/s)。
1.2.3 Western Blot检测P16和P67 phox蛋白表达 药物干预完成后,取每组各3只小鼠的股骨组织进行液氮研磨,将RIPA裂解液加入研磨好的股骨组织中提取蛋白,用BCA试剂盒检测蛋白浓度。提取的蛋白进行SDS-PAGE电泳,待溴酚蓝至分离胶底部时转移到PVDF膜上,凝胶成像系统成像后用Quantity One软件分析灰度值。
1.2.4 RT-PCR检测股骨组织p47 phox、gp91 phox和RANKL mRNA的表达 药物干预完成后,取每组各3只小鼠的股骨组织进行液氮研磨,加Trizol 50~100 g/L,颠倒混匀室温放置30 min。4 ℃下以12 000 r/min离心5 min,弃沉淀,加1/5体积氯仿,充分震荡混匀后置于冰上静置5 min。4 ℃下以12 000 r/min离心15 min,将上清转移至新的1.5 mL EP管中。加入与上清等体积的异丙醇,充分震荡混匀,置于冰上静置10 min。4 ℃下以12 000 r/min离心10min,弃上清,加体积分数0.75的乙醇溶液悬浮沉淀。4 ℃下以12 000 r/min离心5 min,吸除上清,自然晾干后加入10 μL DEPC水溶解RNA。用Nanodrop分光光度计测定RNA浓度,用逆转录试剂盒将mRNA逆转录为cDNA。应用RT-PCR法进行扩增,扩增条件:95 ℃、600 s,95 ℃、10 s,60 ℃、10 s,72 ℃、15 s,共计40个循环;95 ℃、10 s,65 ℃、60 s,97 ℃、1 s。PCR引物及其序列见表1。
1.3 统计学方法
应用SPSS 21.0软件对数据进行统计学分析,
计量数据以±s表示,多组比较采用单因素方差分析,组间两两比较采用LSD法,以P<0.05为差异有显著性。
2 结果
2.1 各组小鼠股骨骨密度比较
与Control组相比较,D-gal组小鼠股骨骨密度明显下降;与D-gal组相比较,AOS干预各组小鼠股骨骨密度明显增加,差异均有统计学意义(F=46.853,P<0.05)。见表2。
2.2 各组小鼠股骨组织中P16和P67 phox蛋白表达比较
与Control组相比较,D-gal组小鼠股骨组织中P16和P67 phox蛋白表达增加;与D-gal组比较,AOS干预各组小鼠股骨组织中P16和P67 phox蛋白表达降低,差异均有统计学意义(F=50.862、156.943,P<0.05)。见图1、表2。
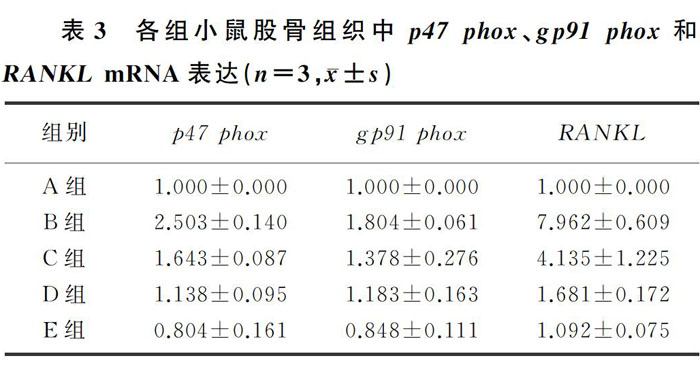
2.3 各组小鼠股骨组织中p47 phox、gp91 phox 和RANKL mRNA表达比较
与Control組相比较,D-gal组股骨组织中p47phox、gp91 phox和RANKL mRNA表达增加;与D-gal组相比,AOS干预各组小鼠股骨组织中p47 phox、gp91 phox和RANKL mRNA表达降低,差异均有统计学意义(F=17.373~112.311,P<0.05)。见表3。
3 讨论
到2050年,65岁以上的老年人口将超过8亿。随着世界人口平均寿命的延长,衰老相关疾病如阿尔茨海默病[9]、骨质疏松[10]等的发病率和死亡率将明显增加,其造成的社会医疗负担也日益加重。骨质疏松症是一种与年龄相关的退行性疾病,严重影响人们的生活质量,导致老年人的死亡率增加。衰老可以引起骨皮质和骨小梁的矿化减少、孔隙度增加及骨密度降低等,导致骨质疏松和骨折的风险增加[11]。本文研究结果显示,D-gal组衰老性骨质疏松模型小鼠的股骨骨密度较Control组显著降低,而与D-gal组相比,AOS干预各组小鼠的股骨骨密度显著增加。说明AOS对D-gal诱导的骨质疏松有保护作用。P16作为细胞周期蛋白依赖性激酶抑制因子[12],在衰老和抑制肿瘤生长过程中起重要作用。P16在大多数啮齿动物和人体组织中的表达随年龄增长而显著增加[13]。本文结果显示,P16蛋白在D-gal组股骨中的表达增加,而与D-gal组相比,AOS干预各组小鼠股骨中P16蛋白的表达降低,说明AOS延缓了D-gal诱导的衰老进程。
氧化应激可诱导DNA损伤和细胞衰老,而抑制衰老可能是治疗骨丢失的有效方法。氧化应激与许多年龄相关疾病(骨质疏松、心血管疾病和神经退行性疾病等)有关,它可以破坏骨骼系统中骨吸收和骨形成的动态平衡,使骨硬度和强度降低,在骨质疏松的发生和发展中起重要作用[14]。ROS的一个主要来源是烟酰胺腺嘌呤二核苷酸磷酸(NADPH)氧化酶,它可促进氧化应激的发生。本研究结果显示,D-gal组股骨中NADPH氧化酶亚基p67 phox、p47
phox和gp91 phox的mRNA表达增加,而与D-gal组相比,AOS干预各组股骨中NADPH氧化酶亚基mRNA表达减少。表明AOS对衰老性骨质疏松的保护作用可能与氧化应激的抑制有关。
随着年龄的增长,破骨细胞介导的骨吸收和成骨细胞介导的骨生成之间失去平衡[15],当骨吸收超过骨形成时出现骨代谢失衡,引起骨量降低从而导致骨质疏松的发生。RANKL在破骨细胞生成中发挥着重要的作用[16],可以与核因子κB受体活化因子(RANK)结合激活信号通路,促进破骨细胞活化、分化和成熟等过程[17]。RANKL相关信号通路被认为是促进骨丢失和破骨细胞活化的主要靶点[18]。本研究结果显示,在衰老性骨质疏松D-gal组股骨中RANKL mRNA表达增加,而与D-gal组相比,AOS干预各组小鼠股骨中RANKL mRNA表达降低,说明AOS对衰老性骨质疏松的保护作用可能与RANKL通路抑制有关。
综上所述,AOS对D-gal诱导的C57BL/6J小鼠骨质疏松有保护作用,其机制可能与氧化应激和破骨细胞活化抑制有关,具体机制还需进一步研究。
[参考文献]
[1]CHEN L R, HOU P, CHEN K H. Nutritional support and physical modalities for people with osteoporosis: current opi-
nion[J]. Nutrients, 2019,11(12):2848.
[2]BOTTANI M, BANFI G, LOMBARDI G.Perspectives on miRNAs as epigenetic markers in osteoporosis and bone fracture risk: a step forward in personalized diagnosis[J].? Front Genet, 2019,10:1044.
[3]ZHANG Zhida, REN Hui, SHEN Gengyang, et al. Animal models for glucocorticoid-induced postmenopausal osteoporosis: an updated review[J].? Biomedicine & Pharmacotherapy, 2016,84:438-446.
[4]LI Y D, HONG Y F, YUSUFUAJI Y, et al. Altered expression of hyperpolarization-activated cyclic nucleotide-gated channels and microRNA-1 and-133 in patients with age-associated atrial fibrillation[J].? Molecular Medicine Reports, 2015,12(3):3243-3248.
[5]LIU Y X, ZUO G L, MENG X, et al. Adrenomedullin inhi-
bits osteoclast differentiation through the suppression of receptor activator of nuclear factor-κB ligand-induced nuclear factor-κB activation in glucocorticoid-induced osteoporosis[J].? Exp Ther Med, 2017,14(5):4009-4016.
[6]GIOVOS G, YAVROPOULOU M P, YOVOS J G. The role of cellular senescence in diabetes mellitus and osteoporosis: molecular pathways and potential interventions[J].? Hormones, 2019,18(4):339-351.
[7]FALKEBORG M, CHEONG L Z, GIANFICO C, et al. Alginate oligosaccharides: enzymatic preparation and antioxidant property evaluation[J].? Food Chemistry, 2014,164:185-194.
[8]DE MARTINIS M, FRANCESCHI C, MONTI D, et al. Inflammation markers predicting frailty and mortality in the elderly[J].? Exp Mol Pathol, 2006,80(3):219-227.
[9]TOEPPER M. Dissociating normal aging from Alzheimers disease: a view from cognitive neuroscience[J]. J Alzheimers Dis: JAD, 2017,57(2):331-352.
[10]FATHI KAZEROONI A, POZO J M, MCCLOSKEY E V, et al. Diffusion MRI for assessment of bone quality: a review of findings in healthy aging and osteoporosis[J].? J Magn Reson Imaging, 2020,51(4):975-992.
[11]JIN H M, WANG Q Q, CHEN K, et al.Astilbin prevents bone loss in ovariectomized mice through the inhibition of RANKL-induced osteoclastogenesis[J].? J Cell Mol Med, 2019,23(12):8355-8368.
[12]ZHEN Y Z, LIN Y J, LI K J, et al. Effects of Rhein lysinate on D-galactose-induced aging mice[J].? Exp Ther Med, 2016,11(1):303-308.
[13]CHEN Linbo, YAO Hui, CHEN Xiongbin, et al. Ginsenoside Rg1 decreases oxidative stress and down-regulates Akt/mTOR signalling to attenuate cognitive impairment in mice and senescence of neural stem cells induced by D-galactose[J].? Neurochemical Research, 2018,43(2):430-440.
[14]ZHU S W, WEI W F, LIU Z W, et al. Tanshinone-ⅡA attenuates the deleterious effects of oxidative stress in osteoporosis through the NF-κB signaling pathway[J].? Mol Med Rep, 2018,17(5):6969-6976.
[15]KIERNAN J, DAVIES J E, STANFORD W L. Concise review: musculoskeletal stem cells to treat age-related osteoporosis[J].? STEM CELLS Transl Med, 2017,6(10):1930-1939.
[16]OMI M, KAARTINEN V, MISHINA Y. Activin A receptor type 1-mediated BMP signaling regulates RANKL-induced osteoclastogenesis via canonical SMAD-signaling pathway[J]. ?J Biol Chem, 2019,294(47):17818-17836.
[17]KWAK S C, BAEK J M, LEE C H, et al. Umbelliferone prevents lipopolysaccharide-induced bone loss and suppresses RANKL-induced osteoclastogenesis by attenuating Akt-c-fos-NFATc1 signaling[J].? Int J Biol Sci, 2019,15(11):2427-2437.
[18]KIM J S, LEE H, NIRMALA F S, et al. Dry-fermented soybean food (Cheonggukjang) ameliorates senile osteoporosis in the senescence-accelerated mouse prone 6 model[J].? Journal of Medicinal Food, 2019,22(10):1047-1057.
(本文編辑 马伟平)
[收稿日期]2019-11-24; [修订日期]2020-05-07
[基金项目]国家自然科学基金资助项目(31571829,31-640050);山东省自然科学基金资助项目(ZR2016HQ23)
[第一作者]王珊(1993-),女,硕士研究生。
[通信作者]毛拥军(1964-),男,博士,教授,博士生导师。
E-mail:mmc168@126.com。
- 潜意识与绘画
- 以画观情
- 拉斐尔前派中的象征性与寓意性
- 西藏工艺美术市场SWOT分析
- 扬州漆艺旅游产品的艺术市场价值探析
- 数字时代的雕塑艺术市场价值分析
- 付玥作品
- 陈煜丹、何岳麟、刘栖宇作品
- 黄浦恩、刘洋、罗玄、彭霞作品
- 刘雪、程垒钏、王海、张子鹏作品
- 高晓燕、黄腾、吴家艳、张琳作品
- 申肖飞作品
- 由“丑书”之争看现代书法
- 张伯英论碑帖临习的意义及方法探析
- 文人篆刻流派的先导
- 绞锋云水注,豪气灿篇章
- 冯超然的绘画人生
- 黑龙江美术多元化发展的条件与对策探索
- 中国油画风景的意象之路
- 中国风景油画的走向
- 中国当代绘画艺术中的女性主义因素
- 改革开放40年“当代工人题材”绘画探究
- “墨点无多泪点多”
- 凛凛岁云暮的心意言说
- 仇英《桃源仙境图》研究
- carriageways
- carried
- carried-away
- carried away
- carried forward
- carried-forward
- carriedforward
- carried on
- carried-on
- carried-out
- carried over
- carriedover
- carrier
- carrier bag
- carrier bags
- carriers
- carriersrisk
- carrier's risk
- carrier-wave
- carries
- carries on
- carrion
- carrions
- carrot
- carroted
- 不递
- 不通
- 不通一窍
- 不通事理行动鲁莽的人
- 不通人事
- 不通人情
- 不通凡卉
- 不通则痛
- 不通大体
- 不通情理
- 不通时宜
- 不通晓
- 不通气
- 不通气儿
- 不通气的烟袋——死心眼儿
- 不通水火
- 不通治乱,不可以语变
- 不通电
- 不通畅
- 不通的
- 不通窍
- 不通道理,不明白事理
- 不通闻问
- 不通音讯
- 不通风