徐木生 何刘 吴深宝 冯萍 徐小宏 陈贵平

[摘要] 目的 研究孟魯司特对大鼠小肠缺血再灌注伤(IIR)肾脏CysLTsR1表达及肾功能的影响。 方法 30只成年雄性wistar大鼠随机分为对照组、缺血再灌注组(模型组)和孟鲁司特组,每组各10只。采用钳闭大鼠肠系膜上动脉建立IIR模型。建模3 h后观察各肾组织病理学形态并评分;免疫组化、Western blot和RT-PCR法检测肾组织CysLTR1和中性粒细胞明胶酶相关脂质运载蛋白表达水平;生化分析仪检测血清尿素氮(BUN)、肌酐(Cr)。 结果 模型组与对照组比较肾组织损伤严重,病理损伤Paller评分明显增高[(408.9±17.3)分 vs (110.3±10.0)分],CysLTR1 mRNA水平显著升高[(3.60±0.84) vs (1.93±0.11)],CysLTR1蛋白表达显著升高,血清BUN和Cr值均显著升高[(21.82±2.39) vs (6.25±0.56);(57.85±8.18) vs (25.55±2.92)],NAGL表达显著升高[(677.67±6.03) vs (576.67±12.66)],差异有统计学意义(P<0.01)。与模型组相比较,孟鲁司特组病理损伤Paller评分显著低于模型组[(323.4±14.9)分 vs (408.9±17.3)分];CysLTR1 mRNA水平显著降低[(2.80±0.36) vs (3.60±0.84)];CysLTR1蛋白表达显著降低(P<0.01);血清BUN和Cr均显著降低[(13.50±1.31) vs (21.82±2.39);(30.85±3.94) vs (57.85±8.18)],差异有统计学意义(P<0.01),NAGL表达降低[(645.00±38.20) vs (677.67±6.03)],差异有统计学意义(P<0.05)。 结论 孟鲁司特可减轻小肠缺血再灌注肾损伤,其机制与其抑制肾组织CysLTR1的活化有关。
[关键词] 半胱氨酰白三烯受体1;孟鲁司特;小肠缺血再灌注;肾功能;中性粒细胞明胶酶相关脂质运载蛋白
[中图分类号] R574? ? ? ? ? [文献标识码] A? ? ? ? ? [文章编号] 1673-9701(2020)32-0040-05
[Abstract] Objective To study the effects of montelukast on the expression of CysLTsR1 and renal function in rats with small intestinal ischemia-reperfusion(IIR) injury. Methods Thirty adult male wistar rats were randomly divided into the control group and the ischemia-reperfusion group (model group), and the montelukast group with ten rats in each group. The IIR model was established by clamping the superior mesenteric artery in rats. After modeling for 3 hours, the pathological morphology of each kidney tissue was observed and scored. The expression levels of CysLTR1 and neutrophil gelatinase-related lipocalin in kidney tissue were detected by immunohistochemistry, Western blot, and RT-PCR methods. The serum urea nitrogen(BUN) and creatinine(Cr) were detected by biochemical analyzer. Results The kidney tissue damage in the model group was more severe than in the control group. The Paller score of pathological injury and the level of CysLTR1 mRNA in the model group was significantly high than that in the control group([408.9±17.3] points vs. [110.3±10.0] points), ([3.60±0.84] vs. [1.93±0.11]). The CysLTR1 protein expression of the model group was significantly increased. The serum BUN, Cr, and NAGL expression of the observation group were significantly higher than those of the control group ([21.82±2.39] vs. [6.25±0.56]; [57.85±8.18] vs. [25.55±2.92]); ([677.67±6.03] vs. [576.67±12.66]), and the difference was statistically significant(P<0.01). The Paller score of pathological damage and CysLTR1 mRNA level in the montelukast group was significantly lower than that in the model group ([323.4±14.9] points vs. [408.9±17.3] points); ([2.80±0.36] vs. [3.60±0.84]). The CysLTR1 protein expression of the montelukast group was significantly lower than that of the model group(P<0.01). The serum BUN, Cr, and NAGL expression of the montelukast group were significantly lower than those of the model group([13.50±1.31] vs. [21.82±2.39]; [30.85±3.94] vs. [57.85±8.18]); ([645.00±38.20] vs. [677.67±6.03]), and the difference was statistically significant(P<0.05). Conclusion Montelukast can alleviate small intestinal ischemia-reperfusion kidney injury. Its mechanism is related to its inhibition of renal activation of CysLTR1.
[Key words] Cysteinyl leukotriene receptor 1; Montelukast; Small intestine ischemia-reperfusion; Renal function; Neutrophil gelatinase-associated lipocalin
肠缺血再灌注伤(Intestinal ischemia reperfusion,IIR)可导致严重创伤、休克、肠系膜动脉血栓形成、绞窄性肠梗阻和小肠移植等常见严重并发症,也可导致肾脏损伤在内的远隔脏器损伤,死亡率高达60%~80%[1],研究表明,核心病理生理变化是IIR时肠道黏膜屏障损伤和血管通透性增加促发肠道菌群移位和机体严重炎症级联反应,大量炎症细胞因子及移位菌群等有害物质通过血液循环损伤肺脏、肝脏和肾脏等远隔脏器,严重者可致多器官功能障碍综合征(Multiple organ dysfunction syndrome,MODS)[2]。肾脏是IIR损伤的一个重要靶器官,临床处理非常棘手,缺乏有效的治疗手段[3]。肾损伤中毒时中性粒细胞明胶酶相关脂质运载蛋白(Neutropil gelatinase-associated lipocalin,NGAL)可早期大量表达于近端小管上皮细胞,参与炎症反应和调节细胞增殖凋亡,可作为急性肾损伤的早期诊断标志物之一[4]。半胱氨酰白三烯(Cysteinyl leukotrienes,CysLTs)是强有力的促炎介质,其配体半胱氨酰白三烯受体1(Cysteinyl leukotriene receptor 1,CysLTR1)广泛表达于炎症细胞和结构细胞表面,在IIR损伤中起重要作用。课题组前期研究结果显示,CysLTR1特异性受体拮抗剂孟鲁司特对IIR模型鼠肠损伤具有保护作用[5-7]。本研究将进一步探讨IIR模型鼠肾损伤CysLTR1的表达及孟鲁司特的保护作用,并探讨其可能的机制,现报道如下。
1 材料与方法
1.1 实验材料
1.1.1 实验动物? 30只雄性wistar大鼠,体重(320±30)g。购自中国人民解放军军事医学科学院实验动物中心,合格证号:SCXK-(军)2007-004。
1.1.2 试剂和仪器? 孟鲁司特片(杭州默沙东制药有限公司,批号:110705);CysLTR1多克隆抗体(Abcam公司,货号:ab95492);NAGL多克隆抗体(Abcam公司,货号:ab137685);SP免疫组化染色试剂盒(福州迈新生物技术开发有限公司);高纯总RNA快速提取试剂盒(Generay公司);SYBR Green PCR试剂盒和逆转录试剂盒(Thermo公司);全自动体生化分析仪(日本日立公司6500型)。
1.2 方法
1.2.1 造模与给药? 采用随机数字表法将大鼠随机分为对照组、缺血再灌注组(模型组)、孟鲁司特组,每组各10只。模型组和孟鲁司特组参照文献建立肠缺血再灌注模型鼠[6],具体如下:建模前各组大鼠禁食12 h,不禁水。孟鲁司特组术前1 h按大鼠2 mg/kg孟鲁司特灌胃给药;对照组和模型组给予等体积生理盐水灌胃。10%水合氯醛(3 mL/kg)腹腔注射麻醉,腹部皮肤常规备皮消毒,取中腹部正中切口长约4 cm,分离肠系膜上动脉(Superior mesenteric artery,SMA)起始部,予无创血管夹夹闭,使之完全阻断血流45 min后松开,再灌注2 h,肠缺血再灌注模型制备成功。夹闭期间断腹腔内注射5 mL生理盐水,以预防松开动脉夹后出现一过性低血容量反应,对照组除不钳夹SMA外其余操作相同。各组关闭腹腔后放回鼠笼观察,自由进食、饮水。再灌注3 h后大鼠再次麻醉进腹,腹主动脉采血后切除大鼠肾脏用于HE染色、RT-PCR、免疫组化和Western blot检测。
1.2.2 大鼠肾脏病理变化及病理损伤评分? 肾脏组织经10%福尔马林固定、包埋、切片后HE染色,光学显微镜下观察肾脏组织病理形态学的变化,每份样本均由两位有经验的病理医师独立病理Paller's评分[7]:每个高倍镜视野下随机选择10个病变的肾小管,按100个肾小管计分,评分标准:肾小管明显扩张、细胞扁平1分;刷状缘损伤1分,脱落2分;管型2分;肾小管管腔内有脱落或坏死细胞(未成管型或细胞碎片)计1分。
1.2.3 大鼠血清标本采集及生化指标检测? 抽取大鼠腹主动脉血液5 mL,2000 r/min離心10 min取血清,采用全自动生化分析仪测定血清尿素氮(Blood urea nitrogen,BUN)和血肌酐(Crea,Cr)含量。
1.2.4 大鼠肾脏免疫组化检测CysLTR1蛋白和NGAL蛋白表达? 大鼠肾脏石蜡切片经二甲苯脱蜡、不同浓度酒精水化和枸橼酸缓冲液抗原修复;分别滴加一抗多克隆CysLTR1抗体(1∶100)和多克隆NGAL抗体(1∶500),4℃过夜;二抗常温孵育30 min;随后DAB试剂染色、苏木素复染及中性树胶封片。显微镜下每张切片随机选取4个高倍镜观察定位,利用Image J计算平均光密度值。
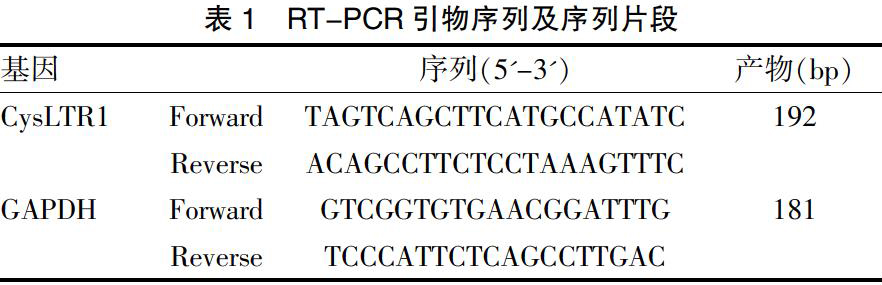
1.2.5 RT-PCR法测定肾脏组织中CysLTR1 mRNA水平? 切取大鼠肾脏组织并迅速放至-80℃冰箱冻存待检。Trizol离心柱法提取组织总RNA,M-MLV逆转录酶进行逆转录,采用荧光定量PCR扩增CysLTR1,以GAPDH为内参,根据Genbank设计引物,由上海生工生物技术公司合成(表1)。荧光定量PCR仪设置参数:95℃预变性l min;95℃变性15 s,55℃退火20 s,72℃延伸20 s,读板,共40个循环;融解曲线。得到每个样品的Ct值,计算每个样品目的基因的相对表达量2-△△Ct。△△Ct=(待测组目的基因平均Ct值-待测组内参基因平均Ct值)-(对照组目的基因平均Ct值-对照组内参基因平均Ct值)。
1.2.6 Western-blot检测大鼠肾脏组织CysLTR1蛋白表达? 冰上研碎大鼠肾组织100 mg后加入RIPA裂解液,超声破碎后4℃,12000 r/min离心15 min,取上清液,测定蛋白浓度,制定标准蛋白曲线,8%SDS-PAGE凝胶电泳分离目的蛋白,然后将蛋白转印至PVDF膜,5%脱脂奶粉封闭PVDF膜1 h,加CysLTR1抗体(1:500)孵育4℃过夜,含吐温缓冲液(PBST)洗脱一抗(10 min×2次),加1:1000稀释HRP标记的二抗,孵育1 h后PBST洗脱二抗(10 min×3次),滴加显影液(NBT/BCTP)显色,发光压片,以GAPDH作为内参照,Image J分析测定灰度值,结果用靶蛋白/GAPDH比值表示蛋白表达的相对水平。
1.3 统计学方法
采用SPSS17.0统计学软件进行分析处理,计量资料用均数±标准差(x±s)表示,组间比较用单因素方差分析,方差齐时采用t检验,方差不齐时采用校正t检验,P<0.05为差异有统计学意义。
2 结果
2.1 各组大鼠肾脏组织的病理学变化
光镜下对照组大鼠肾小球肾小管清晰可见,未见明显的形态学改变;模型组大鼠肾脏可见不同程度的肾小球萎缩变形,肾间质炎细胞浸润,肾小管上皮细胞肿胀空泡变性,管腔变大,甚至肾小管片状出血坏死;孟鲁斯特组也存在上述病理改变,但较模型组明显改善。Paller评分结果显示:模型组显著高于对照组[(408.9±17.3)分 vs (110.3±10.0)分],差异有显著统计学意义(P<0.01);孟鲁司特组显著低于模型组[(323.4±14.9)分 vs (408.9±17.3)分],差异有显著统计学意义(P<0.01)。见封三图3。
2.2 各组大鼠血尿素氮和血肌酐的水平比较
再灌注后3 h,模型组大鼠血清中BUN、Cr含量显著高于对照组,差异有显著统计学意义(P<0.01);孟鲁司特组大鼠血清中BUN和Cr的含量显著低于模型组,差异有显著统计学意义(P<0.01)。见表2。
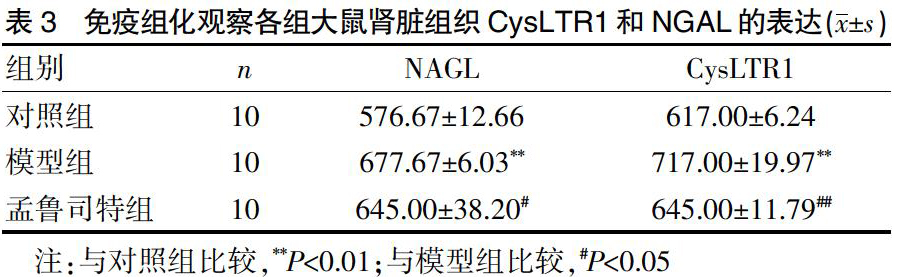
2.3 免疫组化各组大鼠肾脏组织CysLTR1和NGAL的表达
与对照组比较,模型组CysLTR1和NGAL的表达均显著增加,差异有显著统计学意义(P<0.01);与模型组比较,孟鲁司特组CysLTR1表达显著降低,差异有显著统计学意义(P<0.01);NAGL的表达降低,差异有统计学意义(P<0.05)。见封三图4、表3。
2.4 各组肾脏组织中CysLTR1mRNA表达
与对照组相比,模型组CysLTR1 mRNA表达明显升高[(3.60±0.84) vs (1.93±0.11)],两组比较,差异有显著统计学意义(P<0.01);而与模型组比较,孟鲁斯特组CysLTR1 mRNA表达降低[(2.80±0.36) vs (3.60±0.84)],两组比较,差异有显著统计学意义(P<0.01)。
2.5 Western blot观察各组大鼠肾脏组织CysLTR1蛋白表达
各组肾脏组织CysLTR1表达均有阳性条带,位于44 kD。与对照组比较,模型组CysLTR1表达明显增加,两组比较,差异有显著统计学意义(P<0.01);孟鲁斯特组与模型组比较CysLTR1表达明显降低(P<0.01)。见图1。
3 讨论
肠缺血再灌注伤致远隔器官损伤分子机制尚未完全阐明,可能为肠缺血再灌注时活化的血小板和血管收缩介质诱发肠黏膜细胞钙超载和大量氧自由基产生,加剧肠黏膜屏障损伤,肠道细菌和内毒素等有害物质移位至远隔器官[8-10]。肾脏血流动力学具有高灌注和高滤过特性,因此肾脏成为IIR的常见重要靶器官,严重肾脏损伤可致肾功能衰竭而危及生命。半胱氨酰白三烯(CysLTs)包括LTC4、LTD4和LTE4三种亚型,当内脏免疫细胞接触过敏原、炎症细胞因子刺激后,触发细胞内和细胞外液中Ca2+动员,导致胞浆磷脂酶A2(cPLA2)及其他类型PLA2酶的激活,这些酶从膜磷脂中分离出花生四烯酸,然后花生四烯酸通过5-脂氧合酶(5-lipoxygenase,5-LO)在5-LO活化蛋白(5-lipoxygenase-activating protein,FLAP)作用下产生CysLTs[11]。CysLTs除可促进支气管平滑肌收缩外,还主要为促炎因子,与急性或慢性炎症反应有关,主要来源于免疫细胞外,另外内皮细胞吸收白细胞释放的LTA4后,经微粒体GST-2和γ-谷氨酰转肽酶作用也可分泌CysLTs;CysLTsR根据对经典拮抗剂敏感性不同将CysLTs受体主要分为CysLTsR1和CysLTsR2两种亚型。正常人体多种组织细胞如小肠、肝脏和肾脏等器官也低表达CysLTR1,并发挥一定正常的生理功能[12-14]。研究表明,CysLTsR1主要存在于肺部、支气管、平滑肌等组织嗜酸粒细胞、嗜碱粒细胞、中性粒细胞、巨噬细胞、肥大细胞等多种炎症细胞,主要识别LTC4、LTD4和LTE4,其中与LTD4的亲和力最强;而CysLTsR2主要分布在粒細胞。CysLTs通过CysLTs/CysLTsR途径与其他炎症介质相互作用诱发炎症级联放大,增加血管通透性和促进黏液分泌,促进大量炎症细胞聚集[15]。NGAL是由中性粒细胞和某些上皮细胞如肾小管所表达的微量蛋白,缺血性或肾毒性肾损伤时,NGAL在肾脏大量表达,并被释放到尿液和血浆,一般在肾损伤发生后2 h内升高,为早期且敏感的肾损伤生物标志物。本课题组前期研究证实,肠缺血再灌注损伤时小肠组织的CysLTR1表达水平与小肠组织病理损伤程度关系密切,孟鲁司特减轻肠缺血再灌注引起的小肠损伤[5]。为此,本研究进一步研究发现,正常对照组肾脏组织中NGAL和CysLTs轻微表达;当小肠缺血再灌注时肾功能受损严重,肾间质大量炎症细胞浸润、部分肾小球萎缩变形、肾小管细胞水肿甚至出血,肾脏组织CysLTR1蛋白和CysLTR1 mRNA水平表达均明显升高,免疫组化显示CysLTR1主要表达于肾小球和肾小管,而相对应肾小球和肾小管病理损伤严重,NGAL表达量显著增加,表明肾脏组织CysLTsR1蛋白的表达水平和肾脏组织病理损伤及肾脏NGAL水平呈正相关,肾脏组织CysLTsR1蛋白表达水平越高,肾脏组织损伤越严重。孟鲁司特作为选择性可逆的CysLT1受体拮抗剂具有抗氧化抗炎作用,临床上常应用于哮喘的维持治疗、缓解季节性过敏症状[16-17]。本研究结果显示,与模型组相比孟鲁司特组肾组织CysLTR1和NGAL表达显著下降,血清BUN和Cr含量降低,显著改善肾功能,提示孟鲁司特对IIR模型鼠肾损伤具有保护作用,可能与孟鲁司特抑制CysLTs/CysLTsR途径中性粒细胞浸润、抑制分子黏附和脂质过氧化有关[18-20]。
綜上所述,孟鲁司特在IIR模型鼠肾损伤中起保护作用;保护机制是通过抑制肾组织中CysLTsR1表达,抑制CysLTs/CysLTsR途径而减少炎症介质的释放。因此,靶向CysLTsR1有望成为IIR肾损伤新的治疗手段,值得进一步研究。
[参考文献]
[1] Englert JA,Bobba C,Baron RM. Integrating molecular pathogenesis and clinical translation in sepsis-induced acute respiratory distress syndrome[J]. JCI Insight,2019, 4(2):e124 061.
[2] Nadatani Y,Watanabe T,Shimada S,et al. Microbiome and intestinal ischemia/reperfusion injury[J]. J Clin Biochem Nutr,2018,63(1):26-32.
[3] Alexandropoulos D,Bazigos GV,Doulamis IP,et al. Protective effects of N-acetylcystein and atorvastatin against renal and hepatic injury in a rat model of intestinal ischemia-reperfusion[J]. Biomed Pharmacother,2017,89:673-680.
[4] Matsa R,Ashley E,Sharma V,et al. Plasma and urine neutrophil gelatinase-associated lipocalin in the diagnosis of new onset acute kidney injury in critically ill patients[J]. Crit Care,2014,18(4):R137.
[5] Wu S,Zhu X,Jin Z,et al. The protective role of montelukast against intestinal ischemia-reperfusion injury in rats[J]. Sci Rep,2015,5:15 787.
[6] Hu X,Ding C,Ding X,et al. Inhibition of myeloid differentiation protein 2 attenuates renal ischemia/reperfusion-induced oxidative stress and inflammation via suppressing TLR4/TRAF6/NF-kB pathway[J]. Life Ences,2020, 256:117 864.
[7] Shiva N,Sharma N,Kulkarni YA,et al. Renal ischemia/reperfusion injury:An insight on in vitro and in vivo models[J]. Life Ences,2020,256:117 860.
[8] Ceulemans LJ,Verbeke L,Decuypere JP,et al. Farnesoid X receptor activation attenuates intestinal ischemia reperfusion injury in rats[J]. PLoS One,2017,12(1):e0169 331.
[9] Paller MS,Hoidal JR,Ferris TF. Oxygen free radicals in ischemic acute renal failure in the rat[J]. J Clin Invest,1984,74(4):1156-1164.
[10] Grootjans J,Lenaerts K,Buurman WA,et al. Life and death at the mucosal-luminal interface:New perspectives on human intestinal ischemia-reperfusion[J]. World J Gastroenterol,2016,22(9):2760-2770.
[11] Liu Z,Qu M,Yu L,et al. Artesunate inhibits renal ischemia-reperfusion-mediated remote lung inflammation through attenuating ROS-induced activation of NLRP3 inflammasome[J]. Inflammation,2018,41(4):1546-1556.
[12] Otunctemur A,Ozbek E,Cekmen M,et al. Protective effect of montelukast which is cysteinyl-leukotriene receptor antagonist on gentamicin-induced nephrotoxicity and oxidative damage in rat kidney[J]. Ren Fail,2013,35(3):403-410.
[13] Theron AJ,Steel HC,Tintinger GR,et al. Cysteinyl leukotriene receptor-1 antagonists as modulators of innate immune cell function[J]. J Immunol Res,2014,2014:608 930.
[14] Song W,Zhang Y,Wang J,et al. Antagonism of cysteinyl leukotriene receptor 1(cysLTR1) by montelukast suppresses cell senescence of chondrocytes[J]. Cytokine,2018,103:83-89.
[15] Duah E,Teegala LR,Kondeti V,et al. Cysteinyl leukotriene 2 receptor promotes endothelial permeability,tumor angiogenesis,and metastasis[J]. Proc Natl Acad Sci USA,2019,116(1):199-204.
[16] Paolo Gelosa,Elisabetta Bonfanti,Laura Castiglioni,et al. Improvement of fiber connectivity and functional recovery after stroke by montelukast,an available and safe anti-asthmatic drug[J]. Pharmacol Res,2019,142:223-236.
[17] Francis A,Baynosa R. Ischaemia-reperfusion injury and hyperbaric oxygen pathways:A review of cellular mechanisms[J]. Diving Hyperb Med,2017,47(2):110-117.
[18] Malek M,Nematbakhsh M. Renal ischemia/reperfusion injury; from pathophysiology to treatment[J]. J Renal Inj Prev,2015,4(2):20-27.
[19] Gad AM,El-Raouf OMA,El-Sayeh BM,et al. Renoprotective effects of montelukast in an experimental model of cisplatin nephrotoxicity in rats[J]. J Biochem Mol Toxicol,2017,31(12):10.1002/jbt.21979.
[20] Kse E,Oguz F,Vardi N,et al. Therapeutic and protective effects of montelukast against doxorubicin-induced acute kidney damage in rats[J]. Iranian Journal of Basic Medical Ences,2019,22(4):407-411.
(收稿日期:2020-09-17)
- “互联网+”新时代下的大学英语教学策略
- 论PBL在基础护理学教学中的应用分析
- 基于翻转课堂教学法的《微积分》教学改革与实践
- 新形势下高职院校网络安全管理体系构建
- 论新时代现实题材文艺的创作
- 概率统计中积分的特点及独立学院教改方案
- 高效课堂教学案例
- 职高对口单招学生管理现状分析与对策研究
- 农村未成年人阅读服务与社会多元化合作机制研究
- 一种模块化多旋翼无人机的设计
- 二轮驱动差速结构设计
- 8字曲线路径规划凸轮设计
- 基于SMA驱动的扭转弹簧式分离装置设计概述
- CAD/CAM技术在模具设计与制造的应用探究
- 基于UG和Moldflow的塑料头盖模具设计分析
- 一种宿舍用太阳能可折叠晾衣架
- 智能化建筑电气节能优化设计的分析
- LED电视单元模组设计研究
- 嵌入式技术在智能家居系统设计中的应用
- 煤矿井下带式输送机安全运行分析
- 学分银行在社区教育中的应用研究
- 基于交互式图像检索的检索方法研究
- 应用型本科高校实训基地建设探讨
- 浅谈中低压配电网工程造价管理与控制探讨
- “新工科”背景下应用型本科院校人才培养模式探索
- ponderosities
- ponderosity
- ponderous
- ponderously
- ponderousness
- ponderousnesses
- ponder-over
- ponders
- ponding
- ponds
- pondy
- pong
- ponged
- ponging
- pongs
- pong's
- pongy
- ponied
- ponies
- pontificacy
- pontificate
- pontificated
- pontificates
- pontificating
- pontification
- 把道儿划好
- 把道理记在心里
- 把道路堵住
- 把邪恶或敌对势力全部灭掉
- 把酒
- 把酒临风
- 把酒持螯
- 把酒浇愁
- 把酒浇洒在地上表示祭奠
- 把酒论文
- 把醋罐子打烂
- 把里攥
- 把重点放在某方面
- 把重要的和不重要的事物的地位摆颠倒了
- 把金属坯压成一定形状
- 把金钱、地位看得很轻
- 把钓
- 把钢
- 把钢使在刀刃上
- 把钢用在刀刃上
- 把钱存入银行
- 把钱或物等积存起来
- 把钱扔进大河里——连一声响儿也听不见
- 把钱看得比铜盆还大
- 把钱穿在肋条骨上