贾翼 邓晗 马泽刚

[摘要] 目的 探讨大麻素Ⅱ型受体(CB2受体)激活对1-甲基-4-苯基-吡啶离子(MPP+)诱导原代星形胶质细胞炎症反应的影响。方法 培养原代星形胶质细胞,生长状态良好时将其分为Control组、MPP+组、JWH133(CB2受体激动剂)+MPP+组、AM630(CB2受体抑制剂)+JWH133+MPP+组。应用免疫印迹法(Western Blot)检测Control组和MPP+组细胞CB2受体蛋白的表达。应用实时荧光定量PCR(RT-PCR)检测各组环氧化酶2(COX-2)和诱导型一氧化氮合酶(iNOS)基因的表达。结果 与Control组相比,MPP+组的CB2受体蛋白表达上升(F=29.78,P<0.01)。与Control组比较,MPP+组COX-2和iNOS基因的表达明显上调(F=22.59、11.27,q=10.13、5.57,P<0.01);JWH133预处理抑制MPP+诱导的COX-2和iNOS基因表达的上调(q=6.26、4.16,P<0.05);此抑制作用可被AM630所阻断(q=5.34、5.67,P<0.01)。结论 激活CB2受体可抑制MPP+诱导的原代星形胶质细胞炎症反应。
[关键词] 受体,大麻酚,CB2;大麻素受体激动剂;1-甲基-4-苯基吡啶;星形细胞;环氧化酶2;一氧化氮合酶Ⅱ型
[中图分类号] R338.2 ?[文献标志码] A ?[文章编号] 2096-5532(2020)02-0156-05
doi:10.11712/jms.2096-5532.2020.56.071 [开放科学(资源服务)标识码(OSID)]
[网络出版] http://kns.cnki.net/kcms/detail/37.1517.R.20200417.0907.003.html;2020-04-18 15:26
[ABSTRACT] Objective To investigate the effect of cannabinoid 2 receptor (CB2 receptor) on 1-methyl-4-phenylpyridinium (MPP+)-induced inflammatory response in primary cultured astrocytes. ?Methods Primary cultured astrocytes were cultured and divided into Control group, MPP+ group, JWH133 (CB2 receptor agonist)+MPP+ group, and AM630 (CB2 receptor antagonist)+JWH133+MPP+ group when growing well. Western blotting was used to determine the protein expression of CB2 receptor in Control group and MPP+ group. Quantitative real-time PCR was performed to measure the mRNA expression of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS). ?Results Compared with Control group, the MPP+ group had significantly up-regulated CB2 receptor protein expression (F=29.78,P<0.01) and significantly up-regulated COX-2 and iNOS mRNA expression (F=22.59,11.27;q=10.13,5.57;P<0.01). JWH133 pre-treatment significantly inhibited the up-regulated mRNA expression of COX-2 and iNOS induced by MPP+ (q=6.26,4.16;P<0.01), while this was blocked by CB2 receptor antagonist AM630 (q=5.34,5.67;P<0.01). ?Conclusion Activation of CB2 receptor can inhibit MPP+-induced inflammatory response in primary cultured astrocytes.
[KEY WORDS] receptor, cannabinoid, CB2; cannabinoid receptor agonists; 1-methyl-4-phenylpyridinium; astrocytes; cyclooxygenase 2; nitric oxide synthase type Ⅱ
星形膠质细胞是中枢神经系统中数量最多的一类神经胶质细胞,它们在维持细胞外液动力学、调节神经递质代谢、促进神经再生和修复中起重要的作用[1],在帕金森病(PD)的发生发展中也具有重要作用[2]。PD是一种常见的神经变性疾病,其病理特征为黑质致密部多巴胺(DA)能神经元的选择性缺失[3]。神经炎症则是PD神经变性的主要因素之一[4]。在病理情况下,过度活化的星形胶质细胞释放一系列炎性递质,如肿瘤坏死因子α(TNF-α)、白细胞介素1β(IL-1β)、环氧化酶2(COX-2)[5]和诱导型一氧化氮合酶(iNOS)等,可以引起神经炎症[6],并激活小胶质细胞继续释放炎性递质加重神经炎症,从而诱导神经元死亡[7-8]。因此,有效抑制星形胶质细胞的活化和炎性因子的释放对于PD的治疗有重要意义。大麻素Ⅱ型受体(CB2受体)是一种G蛋白耦联受体[9],大量研究表明CB2受体在中枢神经系统疾病的发病中起重要作用[10-11]。关于CB2受体治疗神经炎症反应的研究已经取得了一定进展。有研究证实,WIN55、212-2(合成CB1/2受体激动剂)和JWH133(选择性CB2受体激动剂)[12]通过激活小胶质细胞CB2受体抑制脂多糖[13]和β-淀粉样蛋白[14]诱导的TNF-α和一氧化氮(NO)的产生;在动物实验中激活胶质细胞CB2受体可以通过抑制炎症反应来保护DA能神经元[15-16]。CB2受体不仅在小胶质细胞中表达,在星形胶质细胞和神经元中也有表达[17],但目前缺少关于星形胶质细胞中CB2受体的研究。目前尚不清楚JWH133能否通过选择性激活CB2受体抑制星形胶质细胞的炎症反应。本研究应用1-甲基-4-苯基-吡啶离子(MPP+)制备原代星形胶质细胞的炎症模型,观察JWH133对MPP+诱导的COX-2和iNOS基因表达的影响,以及CB2受体拮抗剂AM630的阻断效应,确定JWH133激活星形胶质细胞CB2受体是否具有抗炎作用,从而为PD的治疗提供新靶点。
1 材料与方法
1.1 实验材料
二甲基亚砜(DMSO)、MPP+购自美国Sigma-Aldrich公司;DEMED/F12培养液购自美国Hyclone公司;青霉素/链霉素溶液购自索莱宝公司;CB2受体激动剂JWH133和抑制剂AM630购自美国Tocris Bioscience公司,用DMSO溶解制成储备溶液(1 mmol/L)置于-20 ℃储存,使用时用培养液稀释至工作浓度;兔源CB2受体抗体购自英国Abcam公司;兔源GAPDH抗体购自博奥森生物技术有限公司;HRP标记山羊抗兔IgG购自联科生物技术有限公司;TRIzol购自南京诺唯赞生物科技有限公司;PCR逆转录试剂盒和SYBR Green购自abm公司;新生SD大鼠购自济南朋悦实验动物繁育有限公司。
1.2 细胞培养及分组
取新生24 h SD大鼠中脑,将其置于DEMED/F12基础培养液中,除去脑膜和血管,用1 000、200、10 μL枪头轻轻吹打使其呈浑浊离散状态,收集至大离心管中。离心,弃上清,加入含体积分数0.10胎牛血清、20 g/L青霉素/链霉素的DEMED/F12培养液,吹打混匀后将细胞接种到培养瓶中,在37 ℃、含体积分数0.05 CO2培养箱中差速黏附处理(倒置放置)30 min,隔天更换新鲜培养液培养7 d以上,待细胞长满瓶底90%以上时,置于37 ℃摇床中以210 r/min振荡16~18 h除去其他胶质细胞,更换新鲜培养液,用胰酶消化法收集细胞,采用特定星形胶质细胞标记物(GFAP)的免疫荧光染色进行鉴定,星形胶质细胞纯度≥95%,进行下一步实验。将原代培养的星形胶质细胞分为Control组(A组)、MPP+组(B组)、JWH133+MPP+组(C组)和AM630+JWH133+MPP+组(D组)。 Control组正常培养;MPP+组加入200 μmol/L的MPP+孵育24 h;JWH133+MPP+组用1 μmol/L JWH133预处理40 min,然后加入200 μmol/L MPP+共孵育24 h;AM630+JWH133+MPP+组用1 μmol/L JWH133和1 μmol/L AM630预处理40 min,然后加入200 μmol/L MPP+共孵育24 h。
1.3 免疫印迹法(Western Blot)检测CB2 受体蛋白的表达
Control组和MPP+组星形胶质细胞经处理后,吸净培养板中的培养液,每孔加入100 μL的裂解液(RIPA裂解液∶PMSF=99∶1),冰上裂解30 min。刮下底部的蛋白置于相应的EP管中,在4 ℃下以12 000 r/min离心20 min,取上清80 μL,用BCA法测定蛋白浓度。每组蛋白的上样量为20 μg,电泳(电压为80 V和120 V)后将蛋白转移至PDVF膜上(转膜电流为300 mA)。用50 g/L BSA封闭液封闭1 h,然后将PDVF膜放入CB2受体一抗溶液中,置4 ℃摇床上孵育过夜。次日用TBST清洗3次,每次10 min,将PDVF膜放入二抗中,室温孵育1 h,重复TBST 清洗。使用ECL显色液处理进行显影。目的蛋白的表达以CB2受体与GAPDH灰度值的比值表示。实验重复5次,取平均值。
1.4 实时荧光定量PCR(PT-PCR)检测COX-2和iNOS mRNA水平
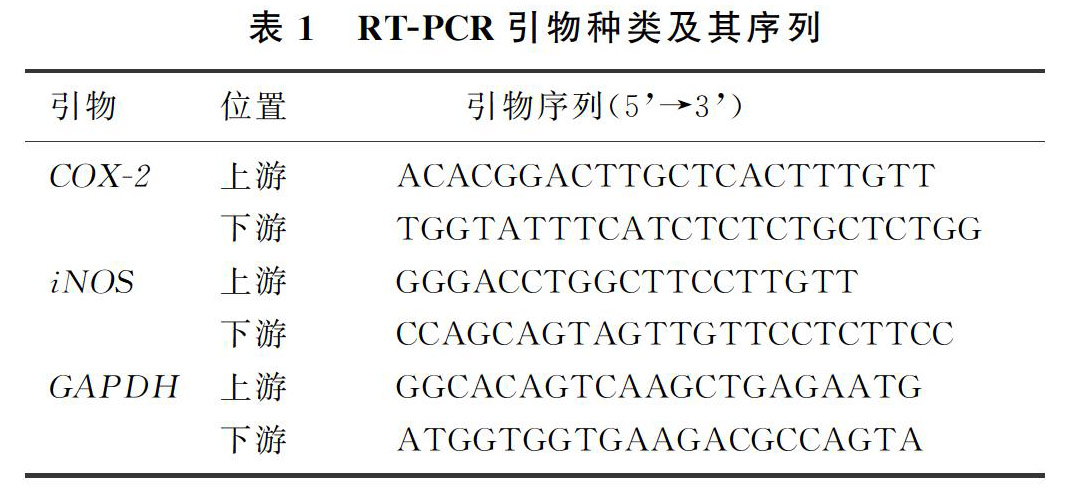
本文4组星形胶质细胞经处理后,采用TRIzol法提取总RNA,将每个样品在1 000 μL TRIzol试剂中匀浆以纯化总RNA,使用反转录试剂盒对2 μg的总RNA进行反转录:加入AccuRT反应混合物(4×)和DEPC水,使总体积达到8 μL,42 ℃变性2 min;加入12 μL的反应体系(内含AccuRT反应混合物终止液2 μL、多合一RT Master Mix(5×)4 μL、DEPC水6 μL) 25 ℃作用10 min,继以42 ℃作用15 min逆转录合成cDNA。采用SYBR Green染料法定量检测COX-2和iNOS的基因表达[18]。大鼠原代星形胶质细胞RT-PCR扩增引物及其序列见表1。采用2-△△CT法计算目的基因相对表达量。实验重复5次,取均值。
1.5 统计学分析
应用Graphpad Prism 5.0软件进行统计学分析,实验所得数据以±s表示,两组间比较采用F检验,多组间的比较采用单因素方差分析(One-way ANOVA),然后用Turkey法进行组间两两比较,P<0.05表示差异有统计学意义。
2 结 ?果
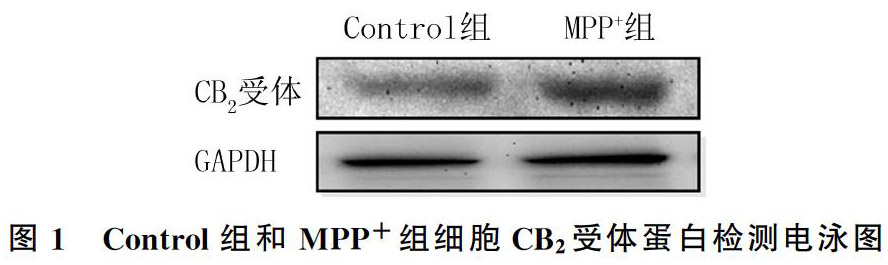
2.1 MPP+处理对原代星形胶质细胞CB2受体蛋白表达的影响
Control组和MPP+组CB2受体蛋白相对表达量分别为0.80±0.08和1.12±0.08,两组比较差异有统计学意义(F=29.78,P<0.01)。表明MPP+处理可以上調原代星形胶质细胞CB2受体蛋白的表达,提示炎症时星形胶质细胞CB2受体表达增多。见图1。
2.2 JWH133对MPP+诱导的原代星形胶质细胞COX-2和iNOS基因表达的影响
与Control组相比,MPP+组COX-2和iNOS基因表达明显上调(F=22.59、11.27,q=10.13、5.57,P<0.01);JWH133+MPP+组细胞COX-2和iNOS基因表达水平较MPP+组均明显降低,差异均具有统计学意义(q=6.26、4.16,P<0.05);而JWH133的抗炎作用可被AM630阻断,AM630+JWH133+MPP+组COX-2和iNOS基因表达水平较JWH133+MPP+组明显升高,差异有统计学意义(q=5.34、5.67,P<0.01)。见表2。
3 讨 ?论
大麻素系统包括内源性大麻素、大麻素受体以及内源性大麻素的合成酶和降解酶。大麻素受体包含大麻素Ⅰ型受体(CB1受体)和CB2受体[7]。大麻素受体与G蛋白耦联,通过抑制腺苷酸环化酶和电压门控钙通道(例如N型、P/Q型和L型钙电流)介导信号转导[9],激活有丝分裂原激活的蛋白激酶(MAPK)和向内整流钾离子通道[19]。与CB1受体调节相比,CB2受体的激活已经被证明没有精神副作用[20]。近年来关于激活CB2受体对神经退行性疾病具有保护作用的研究日渐增多,尤其是针对它的抗炎作用,已有大量研究证据支持CB2受体可以通过参与抑制炎症反应而起到神经保护作用[21-22]。GMEZ-GLVEZ等[23]发现,使用HU-308(一种CB2受体激动剂)激活CB2受体可以抑制脂多糖诱导的纹状体和黑质中炎性因子(例如iNOS)的表达。CHUNG等[16]的研究表明,使用大麻素受体激动剂WIN55、212和JWH133激活CB2受体,可以调节1-甲基-4-苯基-1,2,3,6-四氢吡啶(MPTP)诱导的小胶质细胞活化和炎性因子的表达,从而防止PD小鼠模型中黑质纹状体DA能神经元的死亡。TAO等[24]于2016年发现,JWH133激活CB2受体可以通过促进小胶质细胞表型从M1转至M2,显著抑制炎性因子的释放[25],从而对神经炎症产生影响。JWH133是一种选择性CB2受体激动剂,对CB2受体的选择性是CB1受体的200倍,对CB2受体具有很高的亲和力[11]。本课题组在前期工作中已显示,JWH133激活CB2受体具有对抗MPP+诱导的SH-SY5Y细胞凋亡和神经毒性的作用。关于CB2受体起到的神经保护作用,人们更关注的是CB2受体在小胶质细胞中的抗炎作用,或者CB1/CB2受体在中枢神经系统中的协同工作[26]。但是最近有研究通过免疫组织化学实验分析显示,在PD病人脑中黑质区CB2受体与星形胶质细胞共定位,而不与其他神经胶质细胞或神经元共定位[27]。而且与正常对照组相比较,在PD病人中枢神经系统炎症状态下,星形胶质细胞CB2受体的表达急剧增加[28]。星形胶质细胞在PD进程中炎症所导致的神经元功能障碍和死亡中起关键的作用[28-29],活化的反应性星形胶质细胞释放炎性因子(如IL-1β、TNF-α、COX-2和iNOS)以及活性氧的产生增加[30],会加剧炎症过程,促进PD的发生[7,31]。而抑制活化的星形胶质细胞释放炎性因子将会对PD起到一定的改善作用。
MPP+是MPTP的有毒代謝产物,它可以激活神经胶质细胞、神经元和肥大细胞释放神经炎性递质[32]。本实验应用MPP+制备原代星形胶质细胞炎症模型。Western Blot检测结果显示,MPP+作用于原代星形胶质细胞可引起CB2受体蛋白表达的升高,表明炎症时星形胶质细胞CB2受体表达增多。为了检测CB2受体表达增多是否具有抗炎作用,本研究使用选择性CB2受体激动剂JWH133来激活CB2受体,然后通过检测炎性因子COX-2和iNOS的基因表达水平,探讨JWH133对炎症反应的作用。COX-2是生成前列腺素的关键酶,iNOS可催化生成NO,它们对神经元具有毒性作用,参与并诱导神经炎症,导致神经元细胞死亡[33]。本实验研究结果显示,使用1 μmol/L的JWH133对原代星形胶质细胞进行预处理,能够有效抑制MPP+诱导的COX-2和iNOS基因表达上调,表明选择性激活CB2受体对原代星形胶质细胞具有明显的抗炎作用。进一步使用1 μmol/L的AM630进行验证,结果显示其能够阻断JWH133的抗炎作用。
综上所述,本实验初步证明JWH133激活CB2受体能抑制MPP+诱导的原代星形胶质细胞的炎症反应,抑制COX-2和iNOS基因的表达,其抗炎作用可以被AM630所阻断。本文结果为通过激活CB2受体抑制星形胶质细胞神经炎症从而治疗PD提供了新的方案[34],但还需要进一步研究其所涉及的机制。
[参考文献]
[1] SOFRONIEW M V, VINTERS H V. Astrocytes: biology and pathology[J]. Acta Neuropathologica, 2010,119(1):7-35.
[2] HEATHER D B, WARREN D H, WADE-MARTINS R. The role of astrocyte dysfunction in Parkinsons disease pathogenesis[J]. Trends in Neurosciences, 2017,40(6):358-370.
[3] KORDOWER J H, OLANOW C W, HEMRAJ B D, et al. Disease duration and the integrity of the nigrostriatal system in Parkinsons disease[J]. Brain, 2013,136(8):2419-2431.
[4] HUANG Wanqiu, XU Yazhou, ZHANG Yuxin, et al. Metabolomics-driven identification of adenosine deaminase as therapeutic target in a mouse model of Parkinsons disease[J]. Journal of Neurochemistry, 2019,150(3):282-295.
[5] KEMPURAJ D, THANGAVEL R, YANG E, et al. Dopami-nergic toxin 1-methyl-4-phenylpyridinium, proteins alpha-synuclein and glia maturation factor activate mast cells and release inflammatory mediators[J]. PLoS One, 2015,10(8):e0135776.
[6] LOSSINSKY A S, SHIVERS R R. Structural pathways for macromolecular and cellular transport across the blood-brain barrier during inflammatory conditions. Review[J]. Histology and Histopathology, 2004,19(2):535-564.
[7] DURAISAMY K, THANGAVEL R, GOVINDHASAMY P S, et al. Brain and peripheral atypical inflammatory mediators potentiate neuroinflammation and neurodegeneration[J]. Frontiers in Cellular Neuroscience, 2017,11:216.
[8] BROUGH D, ROTHWELL N J, STUART M A. Interleukin-1 as a pharmacological target in acute brain injury[J]. Experimental Physiology, 2015,100(12):1488-1494.
[9] MUNRO S, THOMAS K L, ABU-SHAAR M. Molecular characterization of a peripheral receptor for cannabinoids[J]. Nature, 1993,365(6441):61-65.
[10] NI R Q, MU L J, AMETAMEY S. Positron emission tomography of type 2 cannabinoid receptors for detecting inflammation in the central nervous system[J]. Acta Pharmacologica Sinica, 2019,40(3):351-357.
[11] CHEN Dejie, GAO Ming, GAO Fenfei, et al. Brain cannabinoid receptor 2: expression, function and modulation[J]. Acta Pharmacologica Sinica, 2017,38(3):312-316.
[12] HUFFMAN J W, HEPBURN S A, LYUTENKO N, et al. 1-Bromo-3-(1′,1′-dimethylalkyl)-1-deoxy-Δ8-tetrahydrocannabinols: new selective ligands for the cannabinoid CB2 receptor[J]. Bioorganic & Medicinal Chemistry, 2010,18(22):7809-7815.
[13] DI MARZO V, STELLA N, ZIMMER A. Endocannabinoid signalling and the deteriorating brain[J]. Nature Reviews Neuroscience, 2015,16(1):30-42.
[14] RAMREZ B G, BLZQUEZ C, GMEZ DEL PULGAR T, et al. Prevention of Alzheimers disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation[J]. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience, 2005,25(8):1904-1913.
[15] JAVED H, AZIMULLAH S, HAQUE M E, et al. Cannabinoid type 2 (CB2) receptors activation protects against oxidative stress and neuroinflammation associated dopaminergic neurodegeneration in rotenone model of Parkinsons disease[J]. Frontiers in Neuroscience, 2016,10(57):321.
[16] CHUNG Y C, SHIN W H, BAEK J Y, et al. CB2 receptor activation prevents glial-derived neurotoxic mediator production, BBB leakage and peripheral immune cell infiltration and rescues dopamine neurons in the MPTP model of Parkinsons disease[J]. Experimental & Molecular Medicine, 2016,48(1):e205.
[17] FERNNDEZ-RUIZ J, ROMERO J, VELASCO G, et al. Cannabinoid CB2 receptor: a new target for controlling neural cell survival[J]? Trends in Pharmacological Sciences, 2007,28(1):39-45.
[18] JIANG Mingchun, CHEN Xiaohan, ZHAO Xia, et al. Involvement of IGF-1 receptor signaling pathway in the neuroprotective effects of Icaritin against MPP+-induced toxicity in MES23.5 cells[J]. European Journal of Pharmacology, 2016,786:53-59.
[19] LU H C, MACKIE K. An introduction to the endogenous cannabinoid system[J]. Biological Psychiatry, 2016,79(7):516-525.
[20] PACHER P, BATKAI S, KUNOS G. The endocannabinoid system as an emerging target of pharmacotherapy[J]. Pharmacol Rev, 2006,58(3):389-462.
[21] RAMIREZ S H, HASKO J, SKUBA A, et al. Activation of cannabinoid receptor 2 attenuates leukocyte-endothelial cell interactions and blood-brain barrier dysfunction under inflammatory conditions[J]. Journal of Neuroscience, 2012,32(12):4004-4016.
[22] ROM S, ZULUAGA-RAMIREZ V, DYKSTRA H, et al. Selective activation of cannabinoid receptor 2 in leukocytes suppresses their engagement of the brain endothelium and protects the blood-brain barrier[J]. The American Journal of Pathology, 2013,183(5):1548-1558.
[23] GMEZ-GLVEZ Y, PALOMO-GARO C, FERNNDEZ-RUIZ J, et al. Potential of the cannabinoid CB2 receptor as a pharmacological target against inflammation in Parkinsons disease[J]. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 2016,64:200-208.
[24] TAO Yihao, LI Lin, JIANG Bing, et al. Cannabinoid receptor-2 stimulation suppresses neuroinflammation by regulating microglial M1/M2 polarization through the cAMP/PKA pathway in an experimental GMH rat model[J]. Brain Behavior and Immunity, 2016,58:118-129.
[25] COLTON C A. Heterogeneity of microglial activation in the innate immune response in the brain[J]. J Neuroimmune Pharmacol, 2009,4(4):399-418.
[26] ZARRUK J G, FERNNDEZ-LPEZ D, GARCA-YBE-NES I, et al. Cannabinoid type 2 receptor activation downregulates stroke-induced classic and alternative brain macrophage/microglial activation concomitant to neuroprotection[J]. Stroke, 2012,43(1):211-219.
[27] NAVARRETE F, GARCA-GUTIRREZ M S, ARACIL-FERNNDEZ A, et al. Cannabinoid CB1 and CB2 receptors, and monoacylglycerol lipase gene expression alterations in the basal ganglia of patients with Parkinsons disease[J]. Neurotherapeutics, 2018,15(2):459-469.
[28] JAYCIE L L, BARKER-HALISKI M L, DAHLE E J, et al. Neuronal injury, gliosis, and glial proliferation in two models of temporal lobe epilepsy[J]. Journal of Neuropathology and Experimental Neurology, 2016,75(4):366-378.
[29] DOMENICO A D, CAROLA G, CALATAYUD C, et al. Patient-specific iPSC-derived astrocytes contribute to non-cell-autonomous neurodegeneration in Parkinsons disease[J]. Stem Cell Reports, 2019,12(2):213-229.
[30] SOFRONIEW M V. Multiple roles for astrocytes as effectors of cytokines and inflammatory mediators[J]. Neuroscientist, 2014,20(2):160-172.
[31] NIRANJAN R. The role of inflammatory and oxidative stress mechanisms in the pathogenesis of Parkinsons disease: focus on astrocytes[J]. Molecular Neurobiology, 2014,49(1):28-38.
[32] DURAISAMY K, SELVAKUMAR G P, THANGAVEL R, et al. Glia maturation factor and mast cell-dependent expression of inflammatory mediators and proteinase activated receptor-2 in neuroinflammation[J]. Journal of Alzheimers Disease, 2018,66(3):1117-1129.
[33] HALD A, LOTHARIUS J. Oxidative stress and inflammation in Parkinsons disease: is there a causal link[J]? Experimental Neurology, 2005,193(2):279-290.
[34] RIETHER D. Selective cannabinoid receptor 2 modulators: a patent review 2009-present[J]. Expert Opinion on Therapeutic Patents, 2012,22(5):495-510.
(本文編辑 马伟平)
- 依托数学问题来打造高效课堂教学
- 基于学生认知规律的思维能力培养
- 引导学生积累数学表象“四部曲”
- 穿插数学游戏 优化数学学习
- 现实数学:让儿童与数学深度遇见
- 把握“四理” 提升素养
- “未来教室”小学数学教学的新时空
- 给力数学实验 发展学生理性思维
- 积累经验:“统计与概率”教学的至真追求
- 策略建构:从意识走向思想
- 小学数学教学中情感素养的有效培养
- 借助数学表象 架起思维桥梁
- 大数据在小学数学教学中的作用
- 重视关键阶段 抓实养成教育
- 谈小学数学问题解决经验在教学中的评价
- 创生小学数学智慧课堂的思考
- 发挥数学史价值,丰富学生全面认知
- 基于学生视角的数学课堂
- 小学生计算能力弱化的原因分析及提升对策
- 玩透“数学实验”,提升数学思维
- 如何培养小学生的数学审题能力
- 低年级数学实验器材开发策略初探
- 曲径可通幽 花儿别样红
- 巧妙用势,成就乘势而上的精彩
- 过程 方法 思维
- reendorsed
- reendorsement
- reendorsements
- reendorses
- reendorsing
- re-endow
- reendow
- reendowed
- reendowing
- reendowment
- re-endowment
- reendowments
- reendows
- reenergize
- reenergized
- reenergizes
- reenergizing
- reengage
- reengaged
- re-engagement
- reengagement
- reengagements
- reengages
- reengaging
- re-engine
- 傲放
- 傲敌
- 傲散
- 傲气
- 傲气损财
- 傲然
- 傲然不屑
- 傲然屹立
- 傲然挺立
- 傲然睥睨
- 傲然矗立
- 傲物
- 傲狎
- 傲狗终归要吃脏布丁
- 傲睨
- 傲睨一世
- 傲睨一切
- 傲睨万物
- 傲睨得志
- 傲睨自若
- 傲纵
- 傲肆
- 傲视
- 傲视一切
- 傲视世间一切,超然物外